Resource Acquisition. Optimal Foraging Theory
Optimal Foraging Theory.How and to what extent have organisms been shaped by natural selection with respect to the efficient acquisition of resources? And are such modifications analogous between macroorganisms and microorganisms? The evolution of search strategies has fascinated ecologists for decades (Hein et al. 2016). In papers published concurrently in the American Naturalist that explored costs and benefits in the economical search for food, Emlen (1966) and, separately, MacArthur and Pianka (1966) launched what became known as ‘optimal foraging theory’ (OFT).
Emlen focused on a mathematical explanation of food preference or selectivity of predators with respect to calories gained and expended, and time invested in the search, capture, and consumption of an item. In their mathematical model addressing the optimal diet of a predator, MacArthur and Pianka addressed where (kinds of habitat patches) and on what items (extent of diet breadth; i.e., specialization) a species would feed if it did so following conventional economic principles of marginal return on investment, thereby optimizing its time or energy budget. MacArthur later expanded his remarks (1972) as has Pianka (2000). The concept has since been applied in numerous contexts (e.g., Pyke 1984; Stephens and Krebs 1986; Stephens et al. 2007). The underlying premise is that natural selection acts to shape energetic costs against gains by foragers: adaptations in attributes such as behavior, physiology, or form that result in a net increase in terms of energy gained should be favored and increase in a population; those that do not should decrease.
Before we turn to the details, brief comment is needed with respect to terminology because the literature on this subject is vast and authors have approached ‘foraging’ either broadly or narrowly. Many papers, especially since the 1990s, consider foraging strictly with respect to the search strategy to find an item only. Much of the foraging literature, particularly prior to the 1990s as illustrated above, considers both the search and the subsequent exploitation of the resource (pursuit, capture, processing). Here we discuss both aspects and begin by focusing on some attributes of the search process. The broader aspects of OFT are discussed subsequently.
The search for resources In The Physics of Foraging, Viswanathan and colleagues (2011) argue that, in their words, “biological foraging is a special case of random searches.” In fact, at the extremes, the search process can be considered to be either random or systematic. Random searches occur only where there is uncertainty (the searcher is uninformed) as to the location of the food source. Here, the search rules involve stochastic processes as depicted in standard random-walk theory (Berg 1993, 2004; Bartumeus et al. 2005) centered on a diffusion process characterized by probabilistic, discrete-step statistical models where displacement events (lengths of movements or steps) are separated by reorientation events (turns).
In the Brownian walk model (named after Brownian motion), step lengths are drawn from an exponential distribution; in the Levy walk or Levy flight model they follow a power-law distribution (de Jager et al. 2011). Simulations show that the latter type results in faster dispersion (hence Levy motion has been called ‘super-diffusive’), less intraspecific competition, and more exploration of new sites than does the Brownian. Levy walks appear to be common in nature and are particularly effective when the resources are scarce and patchily distributed, such as in the open ocean (Humphries et al. 2012; Humphries and Sims 2014). Brownian walks are efficient when prey is abundant and/or relatively homogeneously distributed. Examples of both walks are discussed below.
At the other end of the search spectrum are systematic or deterministic searches. These are effective where the food source distribution is predictable and the location known by the forager. This could occur when sources emit cues, such as a scent trail or nutrient signal, or the forager remembers landscape features and past successes. Such searches include Archimedean spirals or other types of area-restricted patterns (see examples below and Chap. 5 regarding phalanx growth patterns of sessile, modular organisms). Interestingly, simulations show that a Levy walk can become systematized (spiral) by imposing various search restrictions on the walk, such as avoidance of self or self-trails (Sims et al. 2014 and Chap. 5).
With respect to random searches, Levy walks are shared by organisms as disparate as motile bacteria (Reynolds 2015) and albatrosses in their hunt for prey over the ocean (Humphries et al. 2012). Here we focus briefly on bacteria as an example. Motile single cells (typified by most bacteria) can move short distances called ‘runs’ in bacteriology, followed by directional change during ‘tumbles’; the phenomenon usually is illustrated by the model bacterium E. coli (Fig. 3.5a). The biophysics of such patterns has been described in detail by Berg and colleagues in several papers and two books (e.g., Berg and Brown 1972; Berg 1993, 2004).
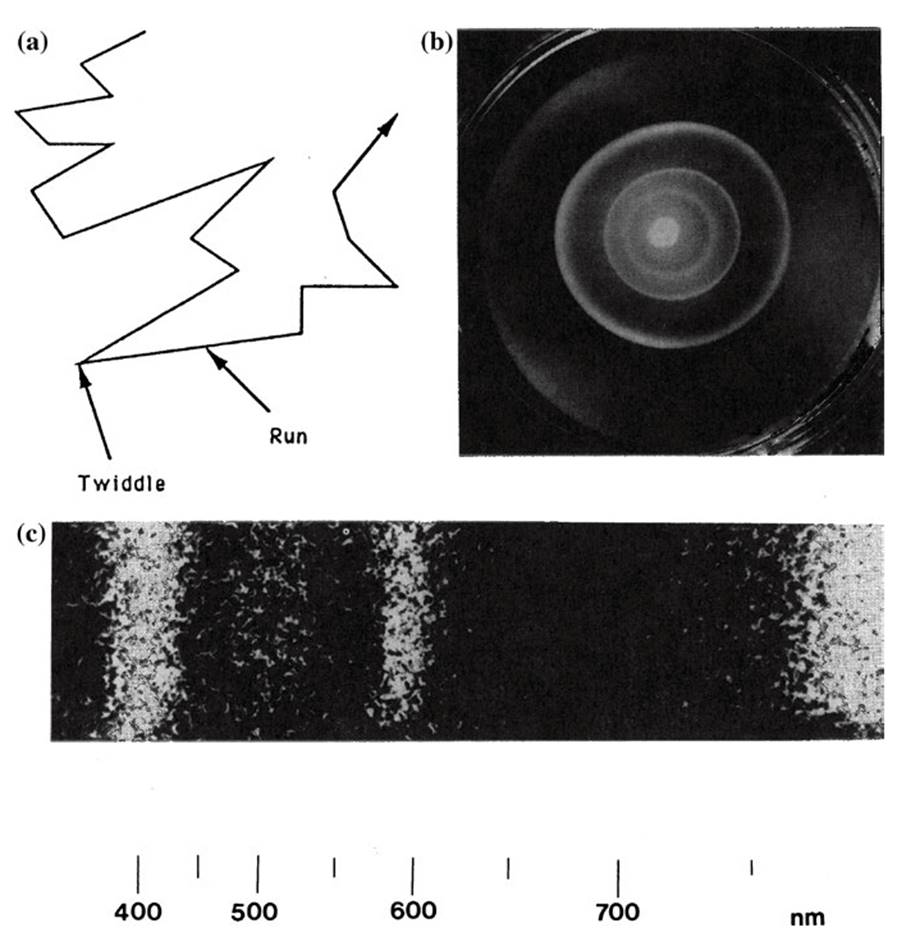
Fig. 3.5. a Idealized random walk of the motile bacterium Escherichia coli in a uniform chemical gradient. Movement consists of brief 'runs' in a particular direction, followed by 'twiddles' during which reorientation occurs. This is only one of several forms of bacterial movement patterns. b If the gradient is nonuniform, directed movement can take place either towards (attractant) or away (repellent) from the source. In this example, bacteria inoculated as a drop in the center of a petri dish have moved outwards as three successive concentric rings, each following a specific nutrient that has become locally depleted. Photo courtesy of Julius Adler, University of Wisconsin-Madison. c Aggregation of individual cells of the phototrophic bacterium Thiospirillumjejense at wavelengths of light at which its pigments absorb. Technically, this particular light-mediated response is an aversion to darkness called scotophobotaxis. Photo courtesy of Norbert Pfennig, University of Konstanz
The actual pattern of swimming varies considerably, however, depending on many factors, including the degree of flagellation and motility adaptations of the species, swimming speed, and characteristics of the habitat (Mitchell 2002; Young 2006; Son et al. 2015). Stocker and Seymour (2012) discuss how some marine bacteria move (in contrast to the enteric bacterium E. coli) and the importance and distinctive attributes of chemotaxis (movement towards or away from a chemical) in the ocean (Fig. 3.5b; see also Mitchell 2002; Mitchell and Kogure 2006; Guasto et al. 2012; for movement in sediments see Fenchel 2008).
Bacterial motility is energetically expensive in terms of the construction and function of the flagellar system (Macnab 1996) suggesting that it has survival value, probably for several reasons (for one example related to resource capture, see Wei et al. 2011). Chemo tactic (Armitage 1999; Eisenbach 2004) as well as two different light-mediated responses exist among bacteria (phototaxis and scotophobotaxis; see Fig. 3.5c and Madigan et al. 2015). In the presence of a chemical, such as a nutrient, the runs become longer and the tumbles less common, so the organism moves up the chemical gradient; similar movements occur away from a source if it is a toxicant. Bacteria can alter their chemotactic search strategies as a function of the energetic status and other properties of the cell and the spatial resource gradient (Mitchell 2002; Mitchell and Kogure 2006).
With respect to systematic searches, and continuing the bacterial theme momentarily, certain bacteria (e.g., some plant- or animal-associated pathogens and the root-nodulating mutualists) also have evolved the ability to respond to signals produced by their specific hosts, which in turn react to their presence, so there is a two-way communication (Brencic and Winans 2005). In at least one extraordinary case, a plant pathogenic bacterium, Agrobacterium tumefaciens, upon gaining entry genetically engineers its specific host to produce new compounds metabolized only by the pathogen. There is a recently described counterpart in medical microbiology where commensal gut bacteria induce formation of fucosylated carbohydrate moieties on intestinal epithelial cells, the fucose then being utilized by the resident beneficial bacteria that confer protection from pathogens (Goto et al. 2014).
Most if not all filamentous fungi can alter their mycelial networks in the search for, or subsequent exploitation of, food resources. Some, such as certain basidiomycetes, can play both a ‘sit and wait’ strategy, analogous to animal predators, by exposing an expansive mycelial network over or into substrate and capturing resources that appear. Alternatively, they can concentrate hyphae into linear explorative and transport organs to move concertedly into new terrain (Boddy et al. 2009; Darrah and Fricker 2014). The energetic trade-offs among reproduction, growth, and foraging have been modeled by Heaton et al. (2016). The mycor- rhizal fungi develop elaborate morphological and physiological relationships within the roots of their plant hosts, to the benefit of both partners (Smith and Read 2008). Biotrophic fungi such as the rusts, powdery mildews, and downy mildews form similar intricate relationships as highly specialized plant parasites (see section Generalists and Specialists, below).
Perhaps even more remarkable than the fungi is the foraging behavior of their distant relatives, the slime molds (Mycetozoa of the phylum Amoebozoa; Fiore-Donno et al. 2010; Yip et al. 2014). Some of these, the myxogastrids or plasmodial slime molds, feed as a single, massive, multinucleate amoeba that tends to be web-like in shape and may be several centimeters in size. Physarum polycephalum forms such feeding networks, adapting its shape to the terrain, moving around obstacles, and responding locally to food patches (Takamatsu et al. 2009; Fricker et al. 2009). It advances generally with an expansive margin that maximizes the area explored but behind the advancing perimeter its conformation is dominated by an array of tubes, through which the cytoplasm streams. Tero et al. (2010) compare the characteristics of this extraordinary biological network to abiotic networks as modeled by the Tokyo rail system. They set up 36 food sources in an array that represented cities in the Tokyo area and compared connections established by foraging Physarum with those of the railway. Remarkably, the exploration and transportation solutions found by the slime mold were mathematically very similar to the rail network in topology, transport efficiency, and tolerance to faults caused by disconnection.
Finally, the strategy of foraging animals after locating a profitable habitat patch often involves modifying their tactics to capitalize on abundant prey. (For discussion of what constitutes a ‘profitable’ patch and how long it may remain so from the consumer’s perspective, see comments on the marginal value theorem in Maynard Smith 1978a; Wajnberg et al. 2000.) Thrushes explore for worms on a lawn by running a short distance, pausing to look about, turning, and making another run. Once food is located, the birds turn more sharply and frequently (Smith 1974; see also Sulikowski and Burke 2011). Gophers search similarly (Benedix 1993). The result of this strategy is concentrated exploration over the zone of high food density, so-called area-restricted searching. Such patterns are not limited to animals but occur among some clonal plants and are a common response among filamentous organisms and organs, from the fungi noted above to the dynamics of root architectures (Chap. 5).
Date added: 2025-06-15; views: 254;
