Versatility as the Ability to Adjust Rapidly
The second interpretation of versatility, speed of response to new (nutrient) conditions, is more amenable to analysis. Prokaryotes as a group have unrivaled metabolic rates (substrate consumed per unit cell mass per unit time) coupled with the ability to react to changing nutrient conditions by rapidly altering metabolic pathways. Thus, what is impressive about these microorganisms is not just the numbers of alternative central and peripheral pathways within a single cell, but that entire peripheral pathways can be rapidly switched on and off. The set of enzymes needed for a particular route is often present or absent as a unit or operon (see below). Details of how these pathways and intermediates change can be found in Neidhardt et al. (1990), Lengeler et al. (1999), Fraenkel (2011). We now look in more detail at some of the mechanistic bases and ecological implications.
Prompt response to ambient nutrient conditions undoubtedly reflects the rapidly fluctuating environmental conditions to which microbes are exposed. These range from excess of a particular substrate to growth-limiting levels or, commonly, to situations where the organism ‘sees’ different but functionally similar substrates in varying concentrations (e.g., both glucose and acetate supply energy and carbon). Enzymes useful at one moment could be useless or detrimental (wasteful) at the next. From the microbe’s standpoint, the premium is on being able to avoid synthesizing catabolic enzymes if it is currently well nourished, while being able to adjust quickly to new resources if the need arises.
Typically, when resources are in high concentration (not growth-limiting), bacteria respond by sequential utilization, with the nutrient supporting the highest growth rate being used preferentially from the mixture (Neidhardt et al. 1990; Lengeler et al. 1999). This phenomenon is known as diauxie and supports the general argument that in bacteria the enzyme machinery adjusts to permit as rapid growth as possible. If substrate concentrations drop to growth-limiting levels, the various nutrients are used simultaneously. Diauxie is one manifestation of the broader regulatory mechanism known as catabolite repression.
Despite some similarities, there are important differences between environmental sensing and gene regulation in prokaryotes and eukaryotes. This topic is discussed in Chap. 7, but it is pertinent to emphasize here some specific aspects that contribute to the nutrient versatility of bacteria. These pertain in part to the direct exposure to the environment in the case of microorganisms, especially those that are unicellular, as opposed to the multicellular and often relatively homeostatic existence of most eukaryotic cells. Thus, the individual cell in a plant or animal body responds to stimuli as mediated systemically by hormones, neurotransmitters, etc., but is buffered from the external environment and also a part of a centrally orchestrated ontogenetic program. The eukaryote cell is constrained by the past conditions, including the developmental program of the organism of which it is a part—an exceptional situation for bacteria (the closest approximations being the few differentiated, multicellular species, or in quorum sensing communications, see Miller and Bassler 2001; and biofilms, see Vlamakis et al. 2013).
In bacteria, fast changes in gene expression are possible because of the kinds of control mechanisms operating at both the transcriptional and translational levels (Fig. 3.7). With respect to the former, new mRNA molecules are produced continuously, with half-lives on average of 0.5-2 min (Lengeler et al. 1999). Even faster control is possible post-transcriptionally to modify enzymes by allosteric and covalent changes, as well as by compartmentation within the cytoplasm. While it may appear wasteful to produce a transcript only to have to modify it later, post-transcriptional regulation allows for fine-tuning. Some attributes of prokaryotes that facilitate rapid response to changing environments are summarized in Table 3.4.
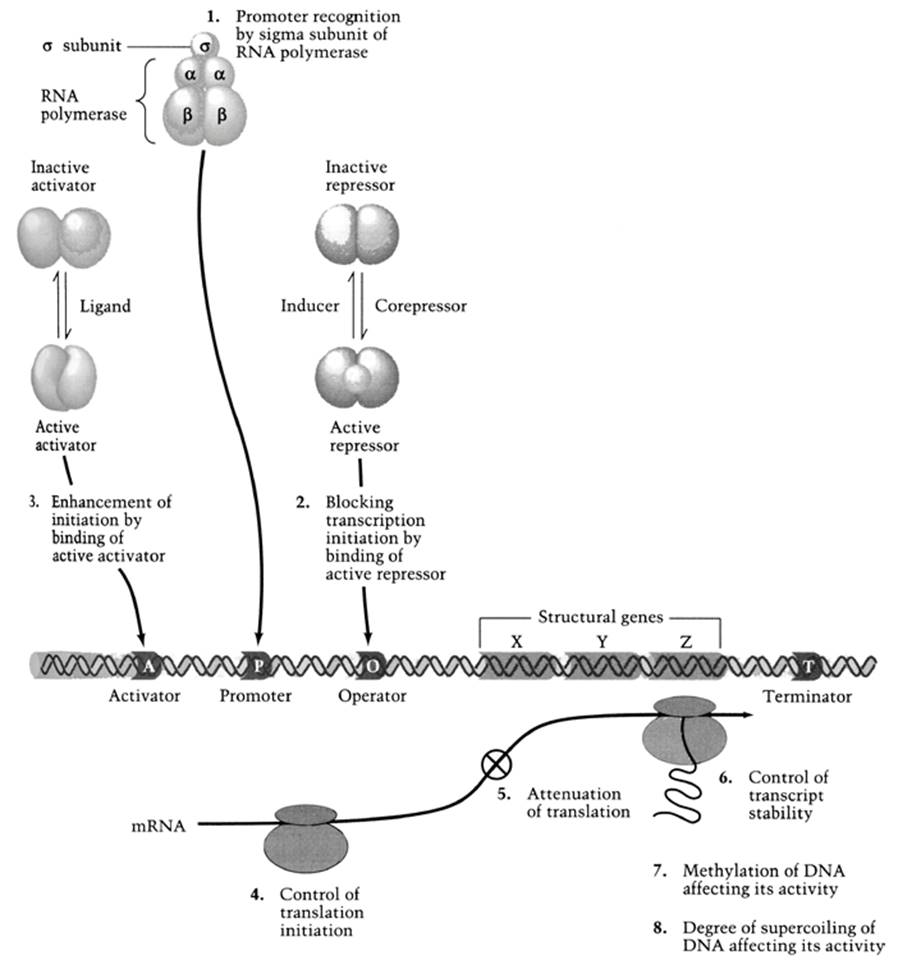
Fig. 3.7. The eight major sites of control for operon expression in bacteria. See also Chaps. 2 and 7. From Neidhardt et al. (1990). Reproduced by permission of Sinauer Associates, Inc., Sunderland, MA © 1990
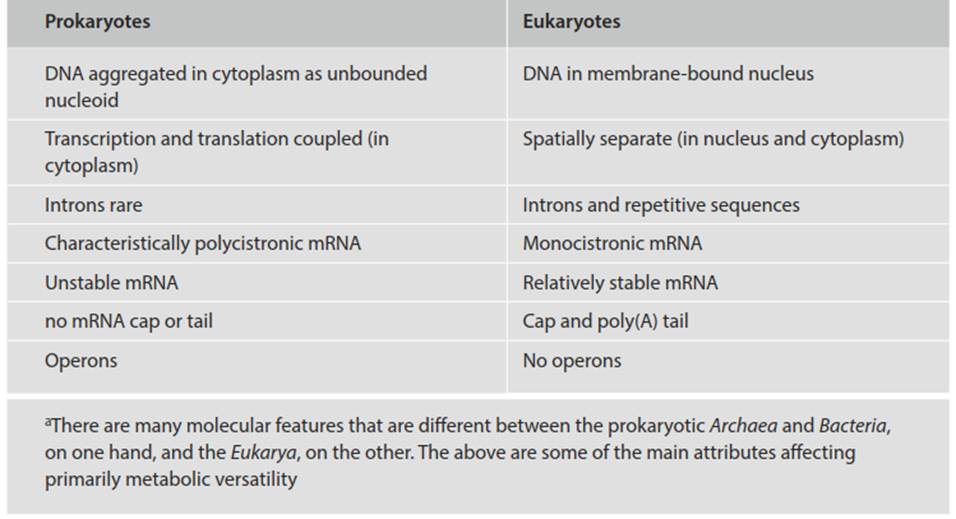
Table 3.4. Some attributes of prokaryotes (cf. eukaryotes) that facilitate rapid response to environmental changesa (Lengeler et al. 1999; Madigan et al. 2015)
These include that their DNA is aggregated as an unenclosed nucleoid within the cytoplasm whereas the chromosomes of eukaryotes are encased within a membrane-bound nucleus. Therefore, external signals affecting nuclear gene expression in eukaryotes must pass through the cytoplasm and then into the nucleus, and likewise, for translation to occur the transcript must move, and be protected from degradation in the process, from the nucleus to ribosomes in the cytoplasm. Bacteria combine transcription and translation, thus expediting the process and doing away with processes involved in transporting the mRNA. Events are not compartmentalized as they are in eukaryotic cells, but rather occur directly in the cytosol. Finally, bacteria exhibit gene clustering, whereby enzymes in a particular pathway may be encoded by adjacent genes. This allows for coordinated regulation of the genes, which are transcribed as one polycistronic mRNA and sequentially translated by ribosomes into each of the proteins.
Probably the classic example of coordinated gene expression is the architecture of the lac region in E. coli, which encodes three genes involved in the metabolism of lactose (Madigan et al. 2015). As alluded to above, the three structural genes are grouped as an operational unit or operon, which is transcribed into a single mRNA molecule. The essence of the mechanism was proposed formally by Jacob and Monod in 1961 (the discovery for which, in part, they received the Nobel Prize in 1965 along with Andre Lwoff, noted in Chap. 2). They postulated that the availability of external food molecules, lactose in this case, in conjunction with the energy status of the cell as mediated through cyclic AMP, control the rate of synthesis of the small inducible enzymes by regulating the synthesis of particular mRNA templates.
Though the basic Jacob-Monod operon model is still valid, transcriptional control has subsequently been shown to be more complex (to involve, e.g., more than just initiation of transcription) and the lac paradigm is not universal. The picture is also different for more complex organisms, where enzymes are coded individually and extensive RNA splicing is involved in the formation of messenger. As an example of comparative speed, translation proceeds about eight times faster in bacteria than in mammals (reviewed in Koch 1971). Transcription is also rapid: the average rate is 48 nucleotides per second, or threefold the step time for incorporation of an amino acid.
In overview, ecological comparisons based on versatility defined as the ability to do many things are ambiguous. While many microorganisms, and plants in general, have the ability to synthesize all their building blocks from a simple carbon source and inorganic ions, animals rely on sophisticated absorption systems for their nutrients from which they synthesize complex macromolecules. When versatility is defined as speed of response to changing nutrient conditions, bacteria are more versatile than macro organisms because they can rapidly alter protein synthesis and entire metabolic pathways. Bacteria can grow at a high rate without excess enzyme ‘baggage’ (Chap. 7), on a preferred nutrient source, under nonlimiting substrate conditions, while retaining the ability to adjust quickly under nutrient limitation by using multiple substrates concurrently or sequentially.
Date added: 2025-06-15; views: 175;
