Case Study: The Gene-for-Gene Interaction and Plant Parasites as Specialists
Nowhere are specialized interactions better illustrated than in the coevolution of parasites and their hosts (Price 1980; Thompson 1994). With respect to some plant pathogen-host relationships in particular, a precise correspondence between genes governing host resistance and those controlling parasite virulence has been well documented, beginning in the 1930s with the classic work that Flor conducted over several decades (e.g., 1956, 1971). He studied the rust disease on flax caused by the biotrophic fungal pathogen Melampsora lini and came to propose the now famous 'gene-for-gene' (GFG) hypothesis that for each gene conferring resistance in the host there is a specific gene conferring avirulence in the pathogen (e.g., Flor 1956; Clay and Kover 1996; Barrett et al. 2009).
Inheritance studies involving pathogen genotypes (races) and plant genotypes (differential varieties of flax) led Flor to conclude that both resistance (R) in the host and avirulence (Avr) in the pathogen are inherited in dominant fashion; specificity is evident in that a resistance reaction only occurs where the pathogen is avirulent and the host is resistant at corresponding loci (Keen 1982; Fig. 3.8). In a strict GFG model, the relevant locus in both host and pathogen is diallelic, with a resistant and susceptible host allele corresponding to a virulence and avirulence allele in the pathogen. If the host is homozygous for the susceptible allele, it will be overcome by all pathogen races, regardless of their virulence genotype; equivalently, a race homozygous for the recessive avirulence allele will infect all host cultivars regardless of their genotype.
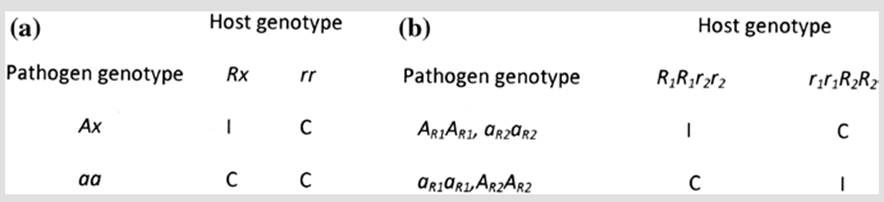
Fig. 3.8. The gene-for-gene relationship as an example of host-parasite specialization. a Quadratic check shown for one diallelic locus in diploid host and diploid or dikaryotic pathogen. The pattern shown here, while consistent with a gene-for-gene relationship, is not definitive because it could also result from general resistance mechanisms. b Reciprocal reactions shown for two loci in host and pathogen. This pattern is diagnostic for gene-for-gene specificity. I incompatible (resistant or no disease); C compatible (susceptible or disease); x = allele unspecified. Each gene of the corresponding pair R1-A1 or R2-A2 is dominant over its defective alleles r1 and a1; r2 and a2. Based on Keen (1982)
A hallmark of GFG interaction is that under parasite pressure, host genotype frequencies oscillate through time in response to corresponding changes in the parasite population. However, Clay and Kover (1996) have argued that a strict gene-for-gene interaction does not lead to cycling of gene frequencies in host and pathogen but rather that typical GFG models actually assume instead a 'matching allele' system. Some GFG models are restrictive with respect to operating assumptions; others do produce cycling in gene frequencies (frequency-dependent selection) if fitness costs to resistance or virulence are assumed (as has been demonstrated in some cases, e.g., Tian et al. 2003; Jones and Dangl 2006). The genetics of resistance and virulence are occasionally more complicated than as assumed in a strict GFG manner but the basic concept has been validated in multiple tests in both agronomic and natural ecosystems (Thompson and Burdon 1992; Lawrence et al. 2007), as well as in some insect-host relationships (Thompson 1994).
If ecological, demographic, and epidemiological considerations are superimposed on the purely genetic GFG model to add realism, various outcomes are possible. For example, Thompson and Burdon (1992) showed that at the local level of a plant population and its immediately associated pathogen population there was little correlation between resistant lines and pathogen races. Instead, locally, the main influence on genetic dynamics was from drift, extinction, and gene flow. At such smaller levels of scale, frequency-dependent selection may still occur but may not be responsible for the pattern in gene frequencies. Most of the GFG models assume panmixis, no drift, and no gene flow, with only mutation and natural selection driving the coevolution of host and parasite population.
The question before us in terms of specialization is how have plant and certain pathogen populations come to be so tightly associated? What is the mechanistic basis for the GFG interaction? In brief, plant resistance to pathogens involves multiple layers of both preformed and inducible responses. The nonspecific, preformed (passive) physical and chemical barriers take such forms as a waxy cuticle overlying the epidermis or toxic peptides, proteins, or other metabolites that deter herbivory and infection.
Through such mechanisms most plants are resistant to most potential pathogens. However, a second line of defense is a relatively specific, generalized basal immunity triggered when plant receptors at the cell surface recognize conserved molecular signals, so-called microbe- associated molecular patterns or MAMPs, characteristic of most microbes. (Interestingly, much of plant-based immunity including the triggering mechanisms of recognition and defense are similar in plants and vertebrates (Staskawicz et al. 2001; Jones and Dangl 2006).
In response to this selection pressure, successful pathogens have evolved various virulence factors such as effector proteins that are translocated into cells to suppress signal detection or host response. These effectors not only influence the virulence of pathogens but are thought to influence host range—some parasites with a narrow range produce host-specific toxins, while those with a broad range have a broad spectrum of phytotoxic molecules, only some of which are operative on any particular host (Barrett et al. 2009).
In certain plant-parasite interactions, the plant responds to virulence factors of the pathogen in a highly specific fashion at the genotype level. Here, the products of resistance (R) genes recognize the structure or activity of pathogen effectors and elicit a classical hypersensitive reaction characterized by localized necrosis and programmed cell death (PCD) of host cells. This GFG response (frequently termed 'effector-triggered immunity,' ETI) provides a specific, qualitative form of resistance to those pathogens that rely on living tissue to grow (obligate biotrophs) because the pathogen also dies and further ingress is halted. It is a form of resistance that allows rapid coevolution of host and parasite because in yet a further turn of the interaction spiral, the pathogen population can respond simply by changing the nature of the effectors being detected or by deploying yet other effectors that suppress the host's ETI. Remarkably, these suppressors themselves have occasionally then become overcome by new R genes. Both the virulence system in the pathogen and the defense system in the host are based on multiple redundancies and so components can be rapidly switched by replacing effectors and modifying resistance genes, respectively. Thus, the cycle of surveillance, detection, and evasion continues indefinitely in a coevolutionary arms race, each member driving the other ever deeper into the realm of specialization (Staskawicz et al. 1995; Schneider and Collmer 2010).
The GFG concept, so thoroughly established by Flor's insightful studies in classical genetics, has since been confirmed and is being increasingly explained by molecular genetics and biochemistry. Several R genes and corresponding avirulence genes have been cloned, sequenced, and their functions inferred (Keen 1990; Bent and Mackey 2007). Most R genes encode proteins of the nucleotide binding, leucine-rich repeat (NB-LRR) type, while avirulence genes typically encode small secreted proteins of known characteristics (Jones and Dangl 2006; Lawrence et al. 2007) that interact with the R proteins. The exact nature of the interactions and how they relate to a PCD response are among the details that remain to be elucidated.
These host-parasite systems not only provide a remarkable example of host-parasite specialization but have important practical implications. A common disease control strategy has been the sequential release of crop varieties carrying single R genes to counter newly evolved races of pathogens (Thompson and Burdon 1992; Hulbert et al. 2001). Molecular approaches will facilitate both the identification of R genes and their potential utilization.
Date added: 2025-06-15; views: 208;
