From Unicell to Multicell; Microorganism to Macroorganism
The first prokaryotes emerging from the progenote presumptively were unicellular (Kaiser 2001) though they may have existed in more-or-less organized colonies (Koonin and Martin 2005). Their nature is obscure because the very early fossil records are nonexistent or largely obliterated for various reasons (Brasier et al. 2006; Schopf et al. 2007). As noted above, to date the first relatively convincing physical evidence of microbial life is from stromatolites suggestive of filamentous and mat-forming cyanobacteria and other forms, followed by evidence for incipient cellular differentiation and diversification about 2 Gya (see comments on Shape below; Nisbet and Sleep 2001; Tomitani et al. 2006; Schirrmeister et al. 2011). So, progression to the ultimately intricate forms of multicellularity really has modest roots among the unicellular and filamentous Bacteria and Archaea (Spang et al. 2015) and, subsequently, the unicellular protists.
Was the primordial shape spherical and if so, why? Although extant prokaryotes are preponderantly unicells, multicellular and in many cases differentiated forms such as the filamentous cyanobacteria, myxobacteria, and actinomycetes have evolved independently multiple times within and among the bacterial clades (Brun and Janakiraman 2000; Flardh and Buttner 2009; Flores and Herrero 2010; Claessen et al. 2014). The longstanding dogma is that bacterial cells are coccoid or rod-shaped and that the primordial prokaryote was spheroidal. Of all possible designs, a true sphere encloses the greatest volume with the least surface area (disadvantageous for transfer processes as we shall see later, but advantageous structurally to restrain turgor pressure). Hence, a spherical shell is robust, spacious, and economical. It seems a logical starting point for elaborations on the architecture of organisms, including the cylindrical form and embellishments thereof (Young 2006; see also Dusenbery 1998), also common among microbes.
Despite their common simplicity of form, however, the extant species of prokaryotes actually span the realm of wondrous cell shapes; inference from molecular phylogenetics suggests the earliest cells were probably rods or filaments and the cocci are a derived mor- photype that arose multiple times as an end-state in various bacterial clades (Siefert and Fox 1998; Young 2006). (Selective forces and bacterial shape are discussed at length by Young 2006.) Even the ‘conventional, unicellular bacteria can modulate growth form advantageously and operate effectively as multicellular organisms in many ways (Shapiro 1998; van Gestel et al. 2015).
Multicellular embellishment of shape. The path to true multicellularity could have arisen by aggregation of cells embedded within mucilage (primordial biofilm); by incomplete cell fission producing chains; or by formation of syncytial filaments, i.e., from an initial condition of a common multinucleated cytoplasm that gave rise subsequently to branching filaments with cross-walls (Claessen et al. 2014). Examples are discussed later in the section Ways to Become Large. It is largely subjective whether a ‘multicellular entity’ such as some of the early or even extant colonial forms really operated as a multicellular individual, as opposed to being little more than a biofilm or an aggregated, more-or-less uncoordinated cell mass of indeterminate shape (for the developmental stages of a contemporary biofilm, see Vlamakis et al. 2013).
The trend to multicellularity (an emergent individual with higher order patterns) was associated with many related and perhaps prerequisite biological changes facilitating coordination and mutual dependency. These changes include such attributes as cell-to-cell adhesion, intercellular connections, differentiation, and communication (and progressively more complex, integrated functions including developmental signaling, complex gene regulatory networks, and programmed cell death in some lineages) (Grosberg and Strathmann 2007; Rokas 2008a, b; Butterfield 2009; Srivastava et al. 2010; Abedin and King 2010). These important changes evidently were staged against a backdrop of multiple forces and converging conditions: biogeochemical, natural selection, genomic, and historical contingency (Carroll 2001; King 2004). Increase in complexity and interdependence is nicely illustrated by the volvocine algae discussed later.
General phylogenetic trends. The major multicellular lineages evidently all arose and developed separately from different unicellular progenitors (Ruiz-Trillo et al. 2007; Abedin and King 2010): For example, land plants (embryophytes) probably originated from a unicellular, flagellated eukaryotic photo autotroph (likely from similar but genetically distinct flagellates that evolved at different times) and subsequently through a multicellular clade of charophyte green algae to the bryophytes (liverworts, mosses, hornworts) (Niklas and Kutschera 2009; Pires and Dolan 2012). The last common ancestor of animals, the so-called Urmetazoan, was multicellular and evolved presumptively from a colonial flagellate, which in turn evolved from a unicellular flagellate, likely a choanoflagellate, the closest known relatives to animals (King 2004; King et al. 2008; Richter and King 2013). Although the origins of the Kingdom Fungi remain more contentious, molecular phylogenies now convincingly support the interpretation that the fungi and animals shared a unicellular opisthokont ancestor (the fungi likely arising from a nucleariid-like amoeboid protist; Steenkamp et al. 2006) and, furthermore, it appears that the incipient animal and fungal lineages subsequently diverged with each having its own unicellular origin (Ruiz-Trillo et al. 2007; Knoll 2011).
Overall, eukaryotic multicellularity is estimated to have arisen independently at least 25 times—sometimes through an intermediary colonial condition (King 2004)—and with occasional reversals (Buss 1987; Grosberg and Strathmann 2007; Niklas 2014). That origins are ‘independent’ has to be inferred, usually from molecular phylogenies supplemented in some cases by evidence from the pattern and placement of cells. Among extant organisms, the multicellular condition appears in at least 16 independent eukaryotic lineages (King 2004; Rokas 2008; Knoll 2011) (Fig. 4.3).
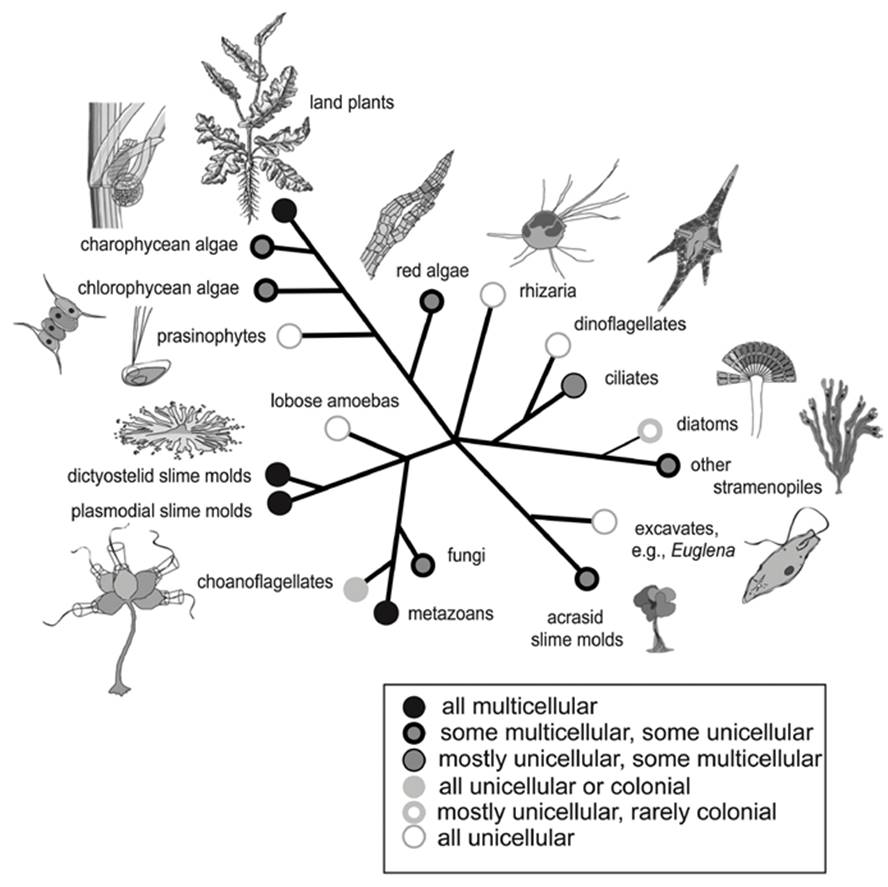
Fig. 4.3. Diverse representation of multicellularity among the major eukaryote clades shown on a simplified, unrooted phylogenetic tree. Some lineages are entirely unicellular or multicellular but most are mixed. For discussion of multicellularity among prokaryotes, see text and Claessen et al. (2014); for discussion of animals, see text and King (2004). From Niklas (2014); figure reproduced from the American Journal of Botany by permission of Karl Niklas and The Botanical Society of America © 2014
A few of these eukaryotes, such as the dimorphic species of fungi, can oscillate between a unicellular and multicellular state dependent on environmental conditions. Others, such as the Myxomycota (including the dictyostelid and acrasid cellular slime molds or social amoebas), as well as the true (plasmodial) slime molds (myxomycetes), have a developmental program featuring both a unicellular and multicellular, differentiated phase. Clues to the origins of multicellularity and incipient differentiation probably reside in some of these borderline, metastable taxa. ‘Metastable’ is used here in the sense that a cell aggregate can differentiate to somatic stalk cells and totipotent spores in a spore-bearing structure (sorocarp) but such states may revert to a multicellular, undifferentiated form with few if any somatic cells (Buss 1982, 1987). (See Tarnita et al. (2015) for some of the genetic and ecological consequences of this extraordinary life cycle.) The remarkable fact is that of the many explorations of the multicellular lifestyle, in only three groups—plants, fungi, and animals—does cellular differentiation occur among many species. Why this major transition to a differentiated entity was closed to all but a few taxa remains a major mystery (Buss 1987, pp. 69-77).
Date added: 2025-06-15; views: 220;
