Evidence for Senescence Among Macroorganisms
Animals. It appears that most if not all animal taxa that reproduce exclusively or primarily sexually senesce (the so-called ovigerous or egg-bearing organisms; Bell 1984; Finch 1990; Ricklefs 2008). This includes the vertebrates and many invertebrates such as the nematodes, rotifers, crustaceans, and insects (unitary organisms in general, Chap. 5). According to Rose(1991, p. 86) “No one has yet found a vertebrate that does not senesce under laboratory conditions when the relevant demographic parameters are measured.”
Interestingly, non-tumorigenic, somatic cells from various animal species (most evidence is from human fibroblasts) grown in vitro have a finite divisional or doubling life span (the famous ‘Hayflick limit’) that correlates loosely in a relative sense with senescence in vivo (Hayflick 1965; Fridman and Tainsky 2008). This appears attributable at least in part to progressive loss of DNA from the specialized termini (telomeres) of eukaryotic chromosomes (see non-evolutionary senescence hypotheses, later). Limited but intriguing evidence suggests that cells undergo fewer doublings when taken from older as opposed to younger organisms, as they do from organisms with shorter as opposed to longer life spans (Goldstein 1974; Rohme 1981). Nevertheless, this correlation is controversial (Rubin 2002) and allegedly ‘neither quantitative nor direct’ (Campisi 2001).
Among invertebrates where clonal growth (asexual reproduction) is prominent in the life cycle, data are either strongly against or equivocal for senescence, at the clonal lineage (genetic individual level; see section Clonal Organisms, below). The strength of the case against senescence in animals appears to depend largely on whether the products of clonal division are essentially morphologically indistinguishable (binary fission) or not. As will become evident later, where a somatic lineage cannot be distinguished from a germ line, or parent from offspring, senescence should not evolve (the classic example is bacteria; among animals the closest parallel would be among certain protozoa; both are discussed later).
Slightly more complicated are cases where division results in products relatively equal in size and requiring only limited development to reach reproductive maturity (paratomical fission; examples include sea anemones, hydroids, planarians, and various oligochaetes). The next step in the gradient is where the asexual offspring are markedly smaller and much less differentiated than the parent, requiring development before becoming an adult (architomical fission; examples include sponges and ribbon worms). This progression implies that senescence should accompany ovigerous reproduction (i.e., where fertilization of eggs is involved), but be relatively negligible for architomical taxa, and absent in paratomical organisms (Bell 1984). Bell provided limited evidence from the culture of six freshwater invertebrates that survival decreased significantly with age in the ovigerous animals (two rotifers plus an ostracod and a cladoceran), but did not change for the two paratomical oligochaetes.
This is consistent with predictions. However, to differentiate clearly in general between para- tomical and architomical categories seems somewhat subjective and arbitrary; it may also be the case that having a soma, however rudimentary, may be sufficient to trigger senescence (Buss 1987). Why the early developmental segregation of germ from soma appears to doom an organism to senescence is not clear. A further complication is that the frequency, timing, and overall importance of asexual reproduction in the life cycle can be expected to vary within a particular taxon depending on the environment (e.g., for marine invertebrates see Hughes and Cancino 1985; Hughes 1989).
The clonal animal, like all clonal organisms, grows indeterminately, in other words without definite restrictions or limits. The ultimate size of the clone is potentially immense as has been discussed previously (7Chap. 5). It is the fate of the clone as the genetic individual that is of evolutionary significance. Here, as is the case with microorganisms discussed below, 'the individual' (implying a physiologically independent individual or ramet) is often confounded with the clone. For example, following on from Bell’s study, Martinez and Levin- ton (1992) ostensibly report senescence in the oligochaete Paranais litoralis, which apparently reproduces almost exclusively in nature by asexual fission. However, the survivorship that they studied was of individual worms, not clones. They also reassess the data of Sonneborn (1930) on survival of the flatworm Stenostomum, where again the focus is on individual, not clonal, survivorship. These and related comments are expanded later, where clonal organisms are discussed as a group.
Plants. Harper’s seminal work on the population biology of plants (e.g., Harper and White 1974; Harper 1977, 1981a) set the stage for all subsequent demographic studies and interpretations of senescence. In pointing out the unique attributes of plants (see also Roach 1993; Munne-Bosch 2015), he questioned Hamilton’s conclusion (1966, p. 12) that “senescence is an inevitable outcome of selection” (see extensive discussion in Evolutionary Theories section, later). Among other things, Harper noted that the reproductive activity of plants can be dictated more by population density than by age of an individual; thus, the age-structured demographic tables so important in animal demography (e.g., Charlesworth 1980, 1994; Caswell and Salguero-Gomez 2013) have limited utility for plants. In general, however, being an older plant means being either the successful larger clone or a bigger tree dominating a canopy.
The difference in seed production between large and small plants (of the same species) may be several orders of magnitude, with a small proportion of plants thus making a grossly disproportionate contribution to fecundity (Levin 1978). While precocious reproduction contributes to reproductive value and is thus favored by selection in animals, it may be so in plants but there are cases where this does not apply and what is important is massive reproduction in later years or just before death (see following comments for trees and semelparous plants). While the old (unitary) animal usually contributes relatively little or nothing directly to population increase, “the oldest individuals in a plant population may have the greatest reproductive output, control the largest fraction of the resources, and control the recruitment of new seedlings” (Harper 1977, p. 700). Thus, it is not surprising that evidence on the occurrence of senescence in plants is mixed and is influenced significantly by factors such as the architecture and life history of a particular species.
The senescence of determinate annual plants after fruit maturation has long been recognized though until recently poorly understood mechanistically (Nooden and Penney 2001; Nooden 2004; Wuest et al. 2016). After a variable period of vegetative growth these plants typically undergo one round of reproduction followed by death within a season, that is, they are monocarpic or semelparous in zoological semantics. Their fecundity rises steeply from zero to a peak and then drops precipitously (Fig. 6.8a). In contrast, the reproductive schedule of almost all perennials is polycarpic (iteroparous; i.e., they undergo multiple rounds of reproduction) because the genet can continue to form new meristems, which themselves reproduce, and so on.
The fecundity schedule depends on whether the perennial has a single meristem or multiple apical meristems. In the former case, as represented by the coconut palm Cocos nucifera, seed production can be expected to rise and then level off for an indefinite period (OFig. 6.8b) (Watkinson and White 1985). Where there are multiple meristems, the outcome depends on whether the plant functions as one physiological individual (aclonal) or several (clonal). In the former case fecundity increases more steeply than for plants with a single meristem; in some systems this increase may be curtailed (Watkinson and White 1985), but most commonly fecundity increases exponentially with size (possibly also with age) as is the case for the clonal plants (Fig. 6.8c, d). Clonal (and to a large degree aclonal perennial) plants are in this sense directly analogous to the clonal marine invertebrates discussed above, and in distinct contrast to the fecundity patterns of unitary organisms that rise early to a maximum and then gradually decline (Charlesworth 1980; Finch 1990).
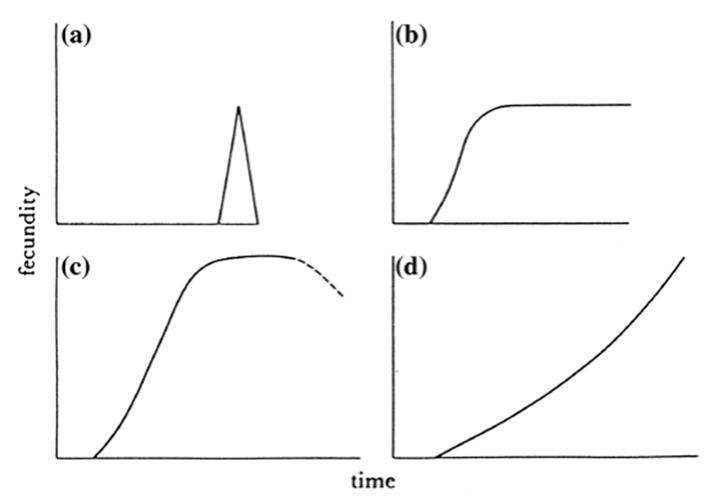
Fig. 6.8. Reproductive schedules (relative fecundity over time) approximated for a semelparous (monocarpic) plants (i.e., those with one round of reproduction, typically annuals); b iteroparous (polycarpic) plants (those with multiple rounds of reproduction, typically perennials) with a single shoot; or with multiple shoots that are c aclonal, or d clonal. From Watkinson and White (1985); reproduced from Philosophical Transactions of the Royal Society of London B by permission of A.R. Watkinson and The Royal Society, ©1985
Similarly, unlike the Hayflick limit, which determines the number of divisions of cultured somatic animal cells noted above, cells taken from plants continue to grow indefinitely in vitro provided that they are subcultured at regular intervals and that growth conditions remain adequate (Murashige 1974; d’Amato 1985). Plant cells are also totipotent (provided that they retain a nucleus and living protoplast), that is, each retains the full genetic/developmental potential of the organism and differentiation is entirely reversible.
The implication of the foregoing is that survivorship rates of genets of semelparous plants are qualitatively similar to those of many unitary organisms (Watkinson and White 1985). In contrast, the indefinite and in general exceptionally long-lived nature of iterop- arous clonal and at least many aclonal plants is akin to that of clonal marine organisms. Evidence for such plants is mixed for occurrence of senescence (summarized by Munne- Bosch 2015). Silvertown et al. (2001) examined 65 species from 24 families of herbs and 12 families of woody plants. Senescence rates, unlike the case for many animals, were independent of the initial mortality rate so the authors used the Weibull function instead of the Gompertz to fit their data.
Some 55% of the plants showed an increase in or maximal value of age-specific mortality late in life that they attribute either to inherent dysfunction or environmental factors, notwithstanding the fact that 61 of the 65 species showed an increasing trend in fecundity. However some caution is warranted in interpreting the synopses. For example, the data for several of the herbs and tree species with increasing age-specific mortality late in life pertained to ramets and not genets. Also, in broad trends, most of the herbs and almost all of the woody species showed a pattern of either asymptotic decrease with time, or a minimum death rate between the youngest and oldest ages.
In the latter case, the pattern of higher mortality in latter stages of life was dominated by the longer lived woody plants (>100 years; see Watkinson and White’s Fig.1). The authors speculate that the short-lived iteroparous perennials die from extrinsic (environmental) causes and the long-lived perennials from physiological deterioration later in life. They conclude that a clonal growth habit is necessary (but insufficient) to prevent the evolution of senescence and that fragmentation of a clone, rather than retention of the clone as an intact unit, is the key determinant. In a fragmented clone, any later disadvantageous trade-offs associated with advantageous early growth or reproduction would tend to be associated with the fragment, i.e., be ramet-directed mortality rather than clonal mortality) rather than the clone as a whole (Silvertown et al. 2001; see later discussion of disposable soma theory).
The work by Silvertown et al. (2001) was extended by Baudisch and colleagues (2011, 2013) in a large study of senescence in 290 angiosperm perennials spanning the gamut in architecture and global distribution (multiple ecoregions). They consider in terms of mortality metrics both patterns in pace and shape. Pace refers here to speed of life measured by life expectancy and categorized as fast, moderate, or slow. This results in short, moderate, or long life, respectively. Shape refers here also to three categories: increasing mortality, designating senescence; decreasing, designating negative senescence; or constant, designating negligible senescence). In principle, species showing any pace of life can exhibit any shape of age-dependent mortality.
The five plant architectures were: (i) cryptophytes (shoot meristems belowground); (ii) hemicryptophytes (shoot meristems near the surface; typically rosette- type plants); (iii) chamaephytes (shoot meristems <25 cm aboveground); (iv) phanerophytes (shoot meristems >25 cm aboveground, typically shrubs and trees); and (v) epiphytes (height determined by their position on the plant, frequently on branches). Pace was related to form, with woody species typically living longer, whereas shape was mainly a function of phylogenetic relatedness. Senescence was observed only among the trees but even here, in the category of phanerophytes, 81% showed negligible or negative senescence. In all, 93% of the plant species did not demonstrate senescence.
The case for trees, broadly speaking, is complicated, with various authors arguing for senescence or absence of senescence. In general, it appears that most species do not senesce, at least not from intrinsic causes the way animals do, but the paucity of long-term demographic studies hampers interpretations, especially for long-lived (hundreds of years) trees (see non-evolutionary theories, below and Roach 1993; Pedersen 1999; Larson 2001; Mencuccini et al. 2005; Petit and Hampe 2006; Stephenson et al. 2014). Even where large and ancient trees become moribund or break in storms, they frequently resprout from adventitious buds (see the extraordinary case for coastal redwoods described in Chap. 7) so life of the genetic individual continues. Meristems (Chap. 5) are the ‘life-blood’ of juvenility for plants (see Chap. 5 and Baurle and Laux 2003; Heidstra and Sabatini 2014; Klimesova et al. 2015; Morales and Munne-Bosch 2015).
Date added: 2025-06-15; views: 223;
