Microorganisms are Not Necessarily r-Strategists
Salisbury’s and Garrett’s hypotheses prompt the more general question of how body size may influence life history traits such as reproductive rate and competitive ability. To what extent do environments influence these traits and what, if any, are the trade-offs? Do microorganisms behave fundamentally differently from macroorganisms? The original and still most general concept that deals with this issue, albeit it indirectly, is r- and K-selection (MacArthur and Wilson 1967; Pianka 1970, 2000; Boyce 1984; Andrews and Harris 1986). MacArthur and Wilson (1963, Chap. 7 in 1967) in articulating their elegant theory of island biogeography considered that at the extremes there were two categories of colonists associated with two different selection regimes.
Where islands are new (untenanted), relatively free of competitors, and with relatively abundant resources, the selection pressure should favor those immigrants that reproduce quickly, i.e., what became known as r-strategists. Over time, as the island becomes increasingly crowded, richer in species, and resource-limiting, selection should favor genotypes that are more competitive or K-strategists.
Consideration of r- and K-selection and the related terminology require first a brief review of population growth. In exponential (unlimited) growth of a closed population (where immigration and emigration can be excluded), the instantaneous change is usually written as dN/dt = (b — d)N, where N is number of individuals and b and d are the average birth and death rates per individual per unit time. Where growth is limited, the logistic equation replaces the exponential and is often written as dN/dt = rN(K — N)/K where r is maximal and fixed and taken to be the difference between instantaneous birth and death rates as population densities approach zero.
Thus, r is usually defined as the intrinsic rate of increase in numbers per unit time per individual and in effect represents the rmax for a specific organism, i.e., the maximum instantaneous rate of increase possible under optimal conditions where r is unconstrained. The rmax for various species is shown in Table 4.4 and discussed by Pianka (1970). At the other extreme, when b = d, the population maintains a stable size and this situation is referred to as the carrying capacity or K. This is the dynamic upper boundary of the population; if N > K the population will decrease; if N < K, the population will increase. Note that in practice as birth and death rates change the actual instantaneous rate of change per individual, ra, is a variable and will change as a function of rmax (the r in the logistic), N, and K.
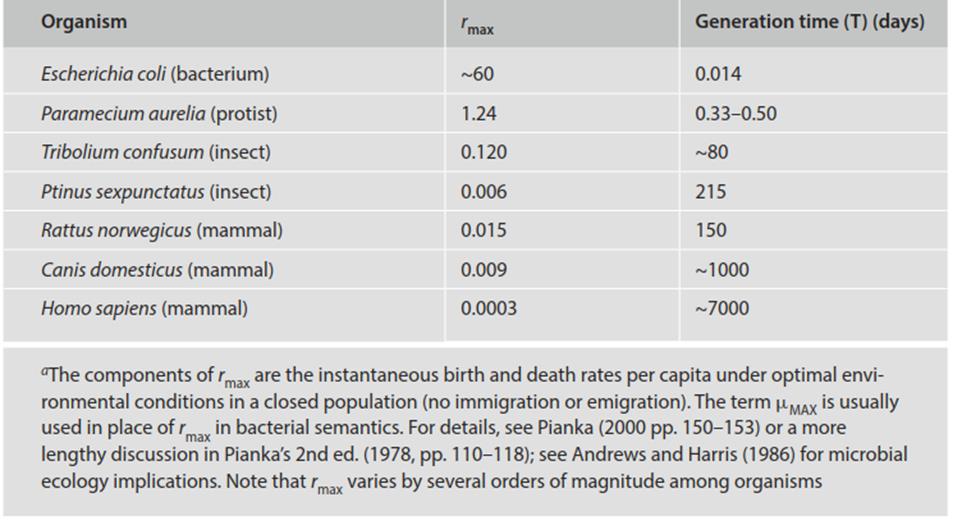
Table 4.4. Estimated maximal instantaneous rates (rmax per capita per day) of increase realizable for various organisms
Thus, the logistic expression describes overall population growth and regulation in a limiting environment. Such growth curves are well known and appear in some form in every introductory ecology and microbiology book as linear (log-transformed) or sigmoid (arithmetic) plots of change in population density versus time. The simplifying assumptions of the logistic expression have long been acknowledged. However, though inherent to the expression, the point is rarely made that the equation is purely descriptive of events, not explanatory. The following discussion summarizes some implications for macroorganism as well as microbial ecology (for details see Andrews and Harris 1986). In particular, the implications of ‘crowding’ should be recognized because, especially in plant and animal ecology, they are often interpreted narrowly to involve only food density, i.e., competition for resources. But crowding implies all factors that change in density-dependent fashion, including parasitism, predation, and—especially in microbiology—production of inhibitory metabolites.
Although both r and K are subject to evolutionary adjustment, the ecological dogma is that a high r occurs at the expense of a low K. In other words, there are inherent trade-offs: an individual cannot maximize both parameters (Roughgarden 1971). From well-controlled experiments with bacterial populations, albeit in non-structured or homogeneous environments (below), there is evidence both for and against the trade-off hypothesis under conditions of resource abundance and scarcity (Velicer and Lenski 1999). This is not surprising in part because whether there is a trade-off is likely trait-dependent. In the formative stages of r/K theory, MacArthur (1972, pp. 226-230), using populations of two alleles, illustrated situations in which r- and K-selection coincided and situations where they did not. He also noted situations where populations could alternate between r- and K-selected environments. Furthermore, a major complication in microbial systems is that in non-structured environments, which are the standard condition in laboratory tests and include suspension cultures and chemostats, a rapidly growing strain will quickly displace a slower competitor before any trade-offs such as between growth rate and growth yield (cells per unit substrate) can be manifested.
An emulsion-based culture system, which imposes a form of structure, may provide for more realistic experimentation, and has led to such trade-offs becoming apparent (Bachmann et al. 2013) (Recall the earlier discussion of growth [ATP] rate versus growth yield in 7Chap. 3. These attributes are relevant here because one attribute of high r is the ability to generate ATP rapidly, if wastefully; a key attribute of a K organism is the ability to harvest resources efficiently.)
The central question is what life history traits are associated with purportedly high r- or K-selecting environments. r-selected individuals are predicted to be smaller in size, to mature earlier, and to have more and smaller progeny. In contrast, K-selected individuals should be larger, show delayed reproduction, allocate resources more to growth (size) for competitive advantage, and hence have fewer but larger offspring. Pianka (1970, 2000) recognized several correlates (Table 4.5; however, see criticism by Boyce 1984).
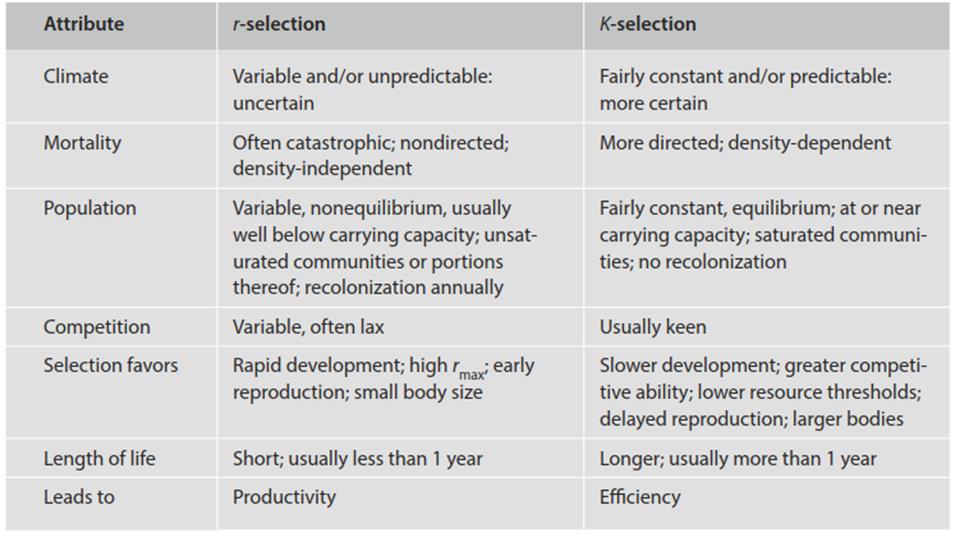
Table 4.5. Some correlates of r- and K-selection (after Pianka 1970)
On balance it is clear from the table that small organisms, microbes in particular, would be shifted toward the r-end of an r - K spectrum. It is important to recognize, however, that microorganisms themselves appear to be relatively r- or K-selected based on their relevant life history traits (Table 4.6; see also Swift 1976; Andrews and Rouse 1982; Andrews and Harris 1986; Andrews 1991).
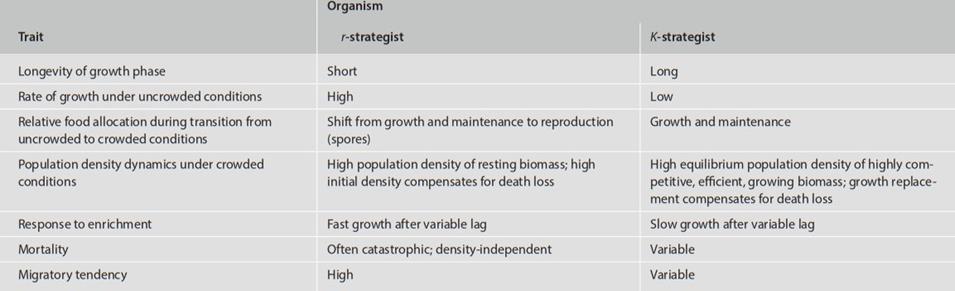
Table 4.6. Some life history features of r- or K-selected microorganisms (Andrews and Harris 1986)
While r- and К-comparisons across widely different taxa can be informative in a general sense, several limitations need to be kept in mind. First, the concept of reproductive value (Chap. 6), and relatedly age-specific models that are familiar to macro-ecologists, are foreign to microbiologists. This is a measure of contemporary reproductive output and residual reproductive value; for macroorganisms it entails life table statistics based on the likelihood of survival and reproduction for specific age classes (juveniles versus adults). A particular life history exhibited by an organism should theoretically be one that has the greatest overall reproductive value. Although the r/К model assumes that environmental fluctuations affect all age classes equally, competing models (e.g., bet-hedging) do not. The issue is moot in microbial ecology because of the short generation times and the plasticity and totipotency of microorganisms, which preclude any analog to adolescence or pre- and post-reproductive classes used in plant and animal biology.
Second is the distinction between modular and unitary organisms (Chap. 5). By growing in modular fashion, the genetic individual or genet can increase indefinitely in size by adding modules (as in flowers, branches, or leaves of a tree; hyphal tips of a fungus). Population growth for these organisms may thus be exponential and, unlike the case for unitary organisms, is not necessarily delayed by postponing reproduction. The predictions from r/К theory for modular as opposed to unitary organisms may be quite different (Sackville Hamilton et al. 1987) as discussed in Chap. 5.
Third, comparisons are best made among species on the same trophic level, where possible, because whether resources or some other mechanism typically acts to limit organisms may depend on their position in the trophic web.
Finally, when organisms are compared, body size should be considered to determine whether natural selection influences reproductive output independently of size. In other words, does selection act primarily on size (Stearns 1983) with a corresponding rate of increase (rmax) following as a consequence? For engineering reasons noted earlier related to complexity, it takes less time to construct a small than a large organism. Small organisms mature and breed earlier than do large organisms, hence the correlation between smaller body size and a higher rmax. Or, alternatively, is selection mainly for a particular rmax, which then dictates a given size because of the relationship between the two parameters (Ross 1988)? Ross examined reproductive patterns in 58 primate species and found generally that after size differences are factored out, the species inhabiting unpredictable, r-envi- ronments had a high rmax.
Selection has evidently acted directly to increase rmax of these species rather than indirectly by decreasing body weight. There was no evidence, however, that either the raw or relative rmax values were as predicted for species in predictable environments. This anomaly remains to be reconciled but may be explained by imprecise classification schemes for the respective environments (Ross 1988). Such formal comparisons have not been made among the microbes. However, because microorganisms of similar size behave very differently in different environments (Andrews and Harris 1986) and also have very different intrinsic growth rates (Brock 1966, p. 95), it seems that selection has been primarily for an r- or К-type strategy rather than indirectly as an unavoidable consequence of size.
The paragraphs above consolidate to this: r- and К-selection can be informative in comparing taxa broadly with respect to life histories along a species continuum, as originally done by Pianka (1970). Such applications are roughly analogous to placing species on a plot of rmax versus generation time as done also by Pianka (1970). The r/К theory does not explain or account for all life history traits and there are organisms that appear not to fit the scheme (see concluding remarks). It has more conceptual value when comparisons are made in a narrower context, such as with respect to strategies among various microbial species
That the logistic equation underpinning r- and К-selection has no mechanistic basis is an obvious limitation. The complexity of most plant and animal systems means that it is difficult if not impossible in population dynamics to delve deeper than gross phenotypic expressions (birth, death, growth). Microbial systems, particularly those with bacteria, can provide for a causative interpretation of the effect of crowding. Subject to the appropriate design noted earlier (e.g., Bachmann et al. 2013), experiments can be conducted under controlled as well as uncontrolled conditions with organisms that are relatively well characterized in terms of their genetics, growth rates and efficiencies, nutritional requirements, and metabolic pathways.
In overview, like many theories in ecology and other sciences, the r/К scheme had a period of ascendancy followed by decline in the wake of critical reassessment; after various refinements, stability occurred at some lower level of influence, with both adherents and skeptics. Disenchantment with the theory (see, e.g., Wilbur et al. 1974; Boyce 1984; Stearns 1977, 1992) has probably stemmed mainly from attempts to overinterpret it. Responses to crowding are one of many that ultimately shape life history traits. Charlesworth (1994, pp. 265-266) has pointed out that the emphasis on logistic growth in formulation of the theory has neglected the consideration of age-structure in populations, thereby omitting some important demographic factors shaping selection. There has been ambiguity in the literature about what r/К is supposed to be describing: response to crowding in terms of resource acquisition only? to density effects broadly? to particular environments broadly?
It is important to recognize that the organism and selective regime attributes identified by Pianka (1970) and others are correlates (as Pianka correctly stated). They do not imply causation. While such caveats were implicit in many of the renditions of r- and К-theory, they were rarely made explicit. As noted here, the theory has not gone beyond phenotypic attributes to address a mechanistic basis for their occurrence. Nevertheless, as a conceptual vehicle for organizing knowledge, describing some general differences among organisms, and prompting tests, the r/К postulate has served us well (though not all ecologists would share this opinion)!
Suggested Additional Reading:
Alegado, R.A. and N. King (Organizers). 2014. The Origin and Evolution of Eukaryotes. Cold Spring Harbor
Perspect. Biol. Doi:10.1101/cshperspect.a016162. Collected papers on the evolutionary transitions to multicel- lularity and complexity.
Bonner, J.T. 1965. Size and Cycle: An Essay on the Structure of Biology. Princeton Univ. Press, Princeton, NJ. A fascinating, eloquent account, timeless in its relevance, of how size affects all creatures with emphasis on how size of the organism changes during the course of the life cycle.
Bonner, J.T. 1988. The Evolution of Complexity by Means of Natural Selection. Princeton Univ. Press, Princeton, NJ. An excellent, stimulating synthesis on why there has been a progressive increase in size and complexity from bacteria to plants and animals.
Carroll, S.B. 2001. Chance and necessity: the evolution of morphological complexity and diversity. Science 409: 1102-1109. An insightful and authoritative synthesis on the evolution of life, including the evolutionary increase in size.
Suggested Additional Reading:
Hedges, S.B. 2002. The origin and evolution of model organisms. Nature Rev. Genet.3: 838-849. An interesting and informative synopsis of times of divergence of the prokaryotes, protists, plants, fungi, and animals, subject to the caveat that all such estimates are works in progress.
Knoll, A.H., D.E. Canfield, and K.O. Konhauser (eds.). 2012. Fundamentals of Geobiology. Oxford Univ. Press, UK. An excellent, well-illustrated synthesis by multiple authors on the early history of life on Earth.
Date added: 2025-06-15; views: 228;
