Some Ecological Consequences of Size. Benefits and Costs
Benefits and Costs.Size effects can be considered at multiple levels—among individuals, populations, or taxa. At the individual level, large size can increase competitive ability, facilitate success as a predator, and deter predation. Harper (Chap. 22, 1977), for example, notes that for plants (unlike most animals) the oldest individuals may be the largest, have the greatest reproductive output, and also control the recruitment of seedlings. Among mobile taxa and within limits, speed tends to increase with size regardless whether the form of locomotion is by running, swimming, or flying (Bonner 1965; McMahon and Bonner 1983; Peters 1983). Interestingly, dolphins move at the same speed (1030 cm/s) as blue whales (Bonner 1965, his Table 2, pp. 185-187). Among land animals there is an eventual trade-off between size and speed because of increasing weight with volume. If swimming speed is expressed in body lengths per unit time (i.e., relative to the amount of new environment sampled by the organism), however, rather than in absolute terms, a motile bacterium explores at the same rate as, say, a dolphin. Large species consume more energy per unit time or distance, but specific costs (i.e., per unit weight) on either basis decrease.
Because generation time increases with size, the individual in a larger bodied species may be more prone to die before reaching sexual maturity and hence leave no descendants. To function, the macroorganism depends on complex developmental programs and integrity of specialized, interrelated cell types, the failure of any one of which could have lethal consequences. Adaptation to change tends to be slower in larger animal species as is repopulation, dispersal ability in general, and the ability to colonize new habitats. Peters (see his Chap. 8, 1983) examined colonization by assuming that a catastrophe depressed populations of micro- and macro organisms to an arbitrarily low (1 gm/km2) density (Fig. 4.12). He asked how long it would take for equivalent recovery of biomass to 100 kg/ km2, assuming each species increased at its maximum intrinsic growth rate (rmax). The time ranged from an order of one day for the bacteria to one century for the large vertebrates. The tendency for body size to increase during evolution is accompanied by higher extinction rates among larger taxa (Chap. 9 in Stanley 1979; for caveats and details see Pimm et al. 1988).
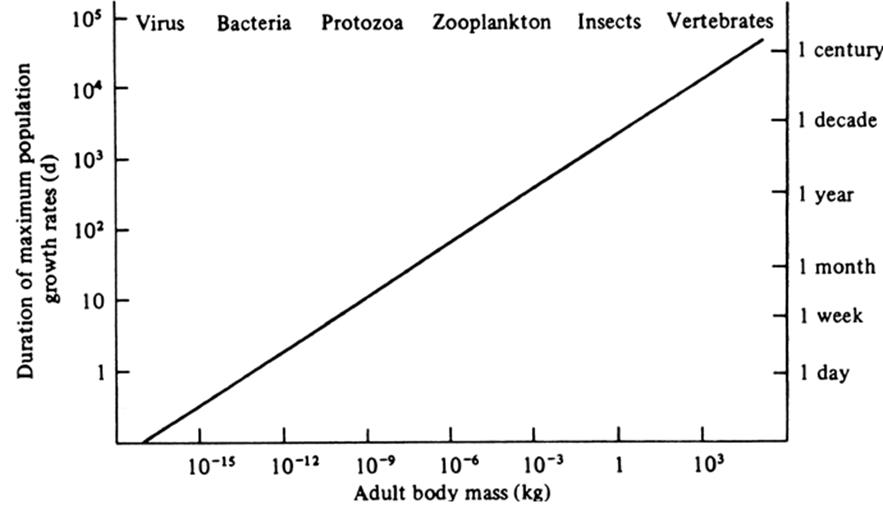
Fig. 4.12. Estimated relative influence of body size on potential duration of maximum (exponential) population growth rates (rmax) and hence of relative colonizing potential of micro- and macroorganisms. For bacteria, it takes on the order of days and for insects on the order of years (right axis) to reestablish a hypothetical population biomass of 100 kg/km2 (average density rounded to the nearest order of magnitude of many animal populations) starting from a negligible starting density of 1 g/km2. From Peters (1983); reproduced from The Ecological Implications of Body Size by R.H. Peters, by permission of Cambridge University Press, ©1983
The mechanisms of extinction are complex and among the most controversial areas of biology. Ultimately extinction is due in varying degrees to intrinsic and extrinsic forces—as Raup (1991) has put it, to bad genes or bad luck. Therefore it is not surprising that understanding why extinction rates should generally be higher for larger species is complicated. But part of the answer likely involves their relative geographic insularity and vulnerability to environmental fluctuations that drive populations down to levels where they can go extinct from demographic stochas- ticity (Fenchel 1993; see ‘The Gambler’s Ruin’ problem, Chap. 3 in Raup 1991). In contrast, species of smaller organisms in general and microorganisms in particular are buffered from such extinction events probably because of their relatively much larger population sizes, capacity for prolonged dormancy, and cosmopolitan distribution. (An interesting postscript is that essentially nothing is known about species extinction rates under natural conditions for microorganisms, especially bacteria.)
Peters’ (1983) recovery time example above also means that although microorganisms respond faster, the duration for which they are able to grow potentially at a maximum rate is orders of magnitude lower than that for the largest vertebrates. The short generation times for microbes that lead rapidly to large population sizes also imply the relatively early onset of crowding effects. The implications for temporal scale in biology are far-reaching, as Peters notes (see also Allen 1977). One consequence is that the apparent relative stability of the larger macroorganisms may be simply because they cannot fluctuate appreciably during the time course chosen for their observation. A wildlife ecologist studying moose, lions, or elephants may make observations at monthly or yearly intervals for decades.
At the other extreme, observations on bacteria or phytoplankton kinetics typically occur over hours, possibly weeks, perhaps for a month or a growing season, depending on the experiment. Choice of the appropriate interval is critical, particularly when interpreting microbial succession on natural substrata. Finally, concepts such as stability or community equilibrium may well be an illusion: they depend on the frame of reference, the scope of the community under scrutiny, the length of life of the organisms concerned, the frequency of the observations, and the duration of the study. It may be that our concept of time is only relevant when the parameters by which we measure it (sun/moon; night/day; seasons) are also responded to by the organism of interest (see Chap. 7).
Some organisms can adjust their size as circumstances dictate. Pufferfish deter predators in part by inflating themselves. Analogously, but in terms of size reduction, vascular wilt fungi can produce a small form of spore that facilitates movement within the conducting vessels of their living hosts, and thereby colonization. Also, because of space limitations, such spores can be smaller (microspores), or in some cases yeast-like cells produced in situ by budding, rather than on conventional stalks or conidiophores (see Sidebar, below). Interestingly, many systemic fungal pathogens of humans and animals are dimorphic (Nemecek et al. 2006), alternating between a single-celled, yeast-like, parasitic state, and a saprophytic mycelial form.
As early as the 1930s Kubiena (1932, 1938) made fascinating observations pertaining to the size of fungi in soil. Some organisms were limited in occurrence to large spaces between soil particles because they were too big to develop in the smaller pores. The fruiting bodies of Cunninghamella occurred only in spaces of diameter exceeding 600 µm. Conidiophores of Botrytis and sporangia of Mucor and Rhizopus were similarly restricted. The sporangio- phores (spore-bearing stalks) of Rhizopus nodosus, normally produced upright, were bent and occasionally spirally curled, molded to fit the space available. Where pores were sufficiently large, soil insects, mites, protozoa, and springtails were found, along with the above molds and a larger fungus, Humicola. Likewise, Swedmark (1964) found the smallest representatives of several phyla in the interstices of marine sand. The larger forms among them were frequently threadlike. Comparable size distribution limits of the filaments of the gliding sulfur bacteria in marine sediment have been reported (Jorgensen 1977), and for percolation of bacteria and fungal zoospores through soil or aquatic sediments (Wilkinson et al. 1981; Fenchel 2008).
In aquatic systems, smallness may also be advantageous for phytoplankton in several ways: by promoting suspension in the photic zone (see earlier comments on Volvox); by increasing the process of light absorption itself; and, because of the increasing surface area:volume ratio with decreasing volume, by fostering nutrient uptake (Fogg 1986; Raven 1986b, 1998; Moore et al. 2013). Small size, irregular shape, and extensive vacuolation increase the area of plasmalemma per unit cytoplasm; these changes provide more sites for nutrient transport into the cell, and tend to reduce the sinking rate.
Cells in the size range of picoplankton (broadly speaking, planktonic organisms between 0.2-2.0 µm; see Fogg for caveats and details) sink at rates that are almost imperceptible (Takahashi and Bien- fang 1983). Phytoplankton sink faster when they are larger, and if turbulence is adequate they will cycle rapidly through the whole water column, scrubbing nutrients as they go. Theoretical calculations show that while a spherical cell of 10 µm in diameter may sink at about 25 cm per day, the rate for a 1-µm cell is 2.5 mm per day (Fogg 1986). Further reduction in size below 1 µm appears to have no additional benefit on sinking rate or light absorption. This example also illustrates that the surface area:volume relationship is especially important for relatively sessile organisms because it directly affects how they forage (Chap. 5), in contrast to the multiflagellated algal and protist components of the plankton.
Although changes in size from small to large or vice versa may carry adaptive benefit for the organism, it is worth noting in passing Gould’s (1966) observation that this does not necessarily apply to specific structures, which must be above a certain minimum size to function at all. There are examples both among the micro- and macroorganisms. To insure effective spore dispersal, mushrooms must be sufficiently high above the ground. The stalks (stipes) of the larger fruiting bodies need not exceed this length and hence on a biomass basis are proportionately smaller than those of the small species (Ingold 1946). Among animals, the size of rods and cones of the eye does not vary with organism size, but is evidently set by optical properties (Haldane 1956; Thompson 1961, pp. 34-35).
Date added: 2025-06-15; views: 249;
