Some Correlates of Size. Complexity
Complexity.To function efficiently, a large organization has many parts and depends on a division of labor or specialization among its component units. This is intuitively true and is evident whether we compare a water flea with a rhinoceros, or a prokaryote with a eukaryote. ‘Complexity’ of organisms is a nebulous, controversial term probably most tangibly based on the number of cell types (which usually have different, specialized functions; Chap. 5 in Bonner 1988; Carroll 2001).
Across the broad sweep of biotic diversity, as well as within specific lineages, life has moved toward increasing complexity: There is a direct correlation between number of cell types and size (Fig. 4.9 and Table 4.2), although for either a given size or number of cell types there is considerable variation in the other parameter. For example, the maximum number of cell types in prokaryotes and protists is about four (prokaryotes are typically of one or two cell types but can reach as many as four in some cyanobacteria; Flores and Her- rero 2010) and rises to about 30 in plants, 50 in nematodes and fruit flies, and over 100 in vertebrates.
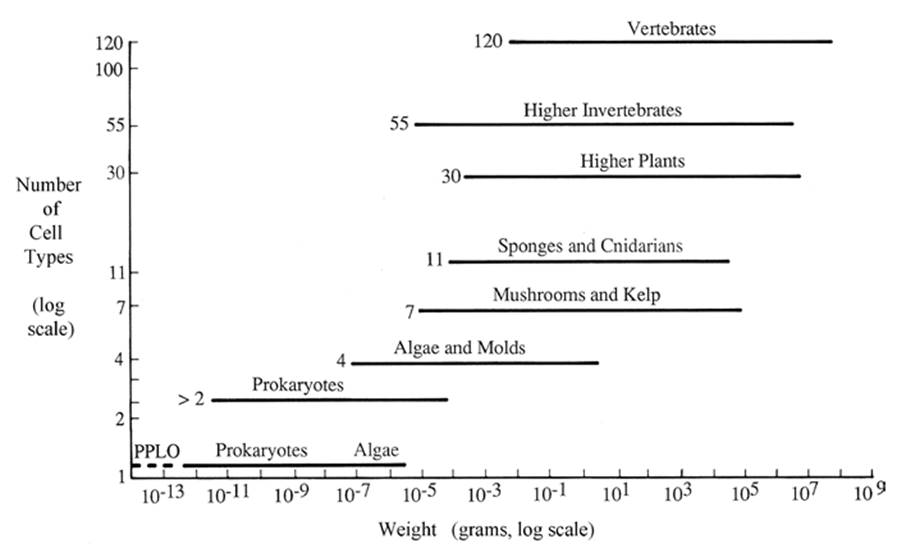
Fig. 4.9. Number of cell types (Y axis) as a function of increasing size (weight). PPLO—pleuropneumonialike organisms are now called mycoplasmas. 'Algae' (atbottom) refers to small cyanobacteria and the green alga Protococcus. The prokaryotes appearing as more than two cell types (second horizontal line from the bottom) include the large cyanobacteria, the spore-formers, and other multicellular bacteria. From Bonner (1988); reproduced from The Evolution of Complexity by Means of Natural Selection by John Tyler Bonner, by permission of Princeton University Press, ©1988
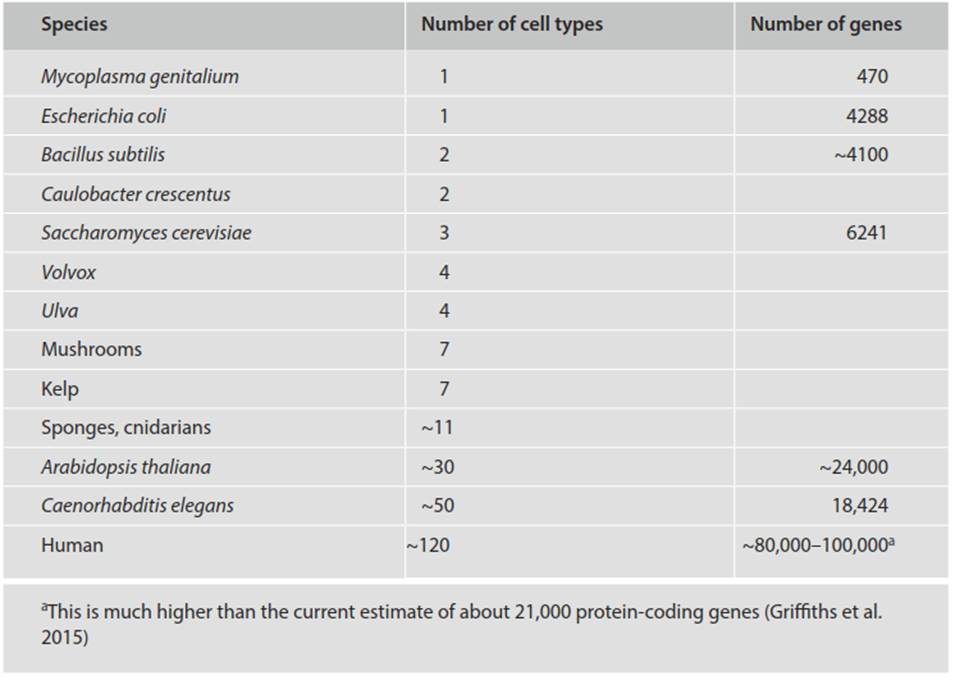
Table 4.2. Number of cell types and number of genes in various organisms (abbreviated from Carroll 2001)
Gene number (also shown in Table 4.2) is sometimes used as a measure of complexity, but is subject to variation from gene duplications and losses, can be difficult to determine accurately, and has several limitations, so is not particularly informative (Carroll 2001). While it is broadly true that eukaryotes have more genes than prokaryotes, gene number varies roughly 10-fold even among prokaryotes, and among eukaryotes the organisms with fewer cell types may have appreciably more genes. Nevertheless, the evolutionary transition to increased size and complexity runs in tandem with phylogenetic increase in nuclear DNA content. For instance, as is well known, the amount of haploid DNA in mammalian cells is, to an order of magnitude, about 103-fold greater than that of bacteria (summarized in Watson et al. 2008, p. 139). These values are approximate and the correspondence between DNA content and complexity is not perfect—some amphibians have 25 times the amount of DNA per cell than do mammals.
The pattern must be more than coincidental and suggests that, in general, gene amplification played a major role in phylogenetic change (Chap. 2 and Stebbins 1968; Griffiths et al. 2015). By virtue of providing an extra gene copy for evolutionary ‘experiments’, duplications foster flexibility and allow novelty to arise in formerly tightly linked traits and pathways (Vermeij 1999). Within phyla, however, there is no correlation between DNA content and complexity. Stebbins (1968) attributed this apparent anomaly to the possibility that the origin of new phyla and the associated requirement for new cells and organs necessitate an increase in different enzyme systems. In contrast, evolution within a phylum may be more a matter of integration of function or alteration of conformation, processes that could be accomplished by mutation and recombination of existing genes.
Probably the most important event spurring evolution of greater complexity arguably was the transition from unicellularity to multicellularity, discussed earlier. Unicells can differentiate only in time, not in space, i.e., they can, at a given time, only be one thing or another, unlike even a two-celled entity where the two members can be structurally or functionally different from one another simultaneously. Once multicellularity arose, it was comparatively easy to embellish rudimentary division of labor and specialization into increasingly sophisticated forms. As these entities and partnerships evolved, new interactions and mutualisms at multiple levels became possible in ways that were not available to their precursors (Vermeij 1999). Another major event advancing complexity was the transition from an aquatic to a terrestrial existence. This led to the embellishment of animal body plans already established in the oceans and to a major radiation of plant life leading to, for example, massive arboreal forms and seed plants. For a fascinating discussion of these points, see Raff (1996 for animals) and Corner (1964) or Graham (1993 for plants).
As the number of distinct parts increases so does the number of different interactions among them. Natural selection can act on size, shape, or complexity, yet a significant change in one influences the others (Bonner 1988, p. 226). Bonner notes that selection acting on complexity is probably more important than that acting on size because increase in number of cell types opens the way for a large increase in size. In contrast, while size alone is somewhat plastic, its upward movement in the absence of an associated increase in complexity is limited by losses in efficiency. One of the implications of the size-complexity issue is that a large organism is locked into an intricate developmental pattern, each step of which is influenced and in many cases predetermined by what has gone before (recall comments in Chap. 1 about ontogenetic constraints). Microorganisms, being small and less complex in number of cell types, escape this complicated ontogeny.
A body plan built on specialized, interdependent subunits offers the benefits of high efficiency at particular tasks (e.g., sight, taste, translocation, support, and defense) at the cost of vulnerability, often death, if an intricate system fails. Such failure occurs, for instance, when tetanus exotoxin binds specifically to one of the lipids of human nerve synapses in the central nervous system; when T-4 lymphocyte helper cells are killed by the AIDS virus; or when propagules of the fungus causing Dutch elm disease block water-conducting xylem vessels of the host. Organs can fail also for hereditary and environmental reasons. By having fewer and less complicated parts, microbes lack the advantages but avoid the shortcomings of life based on a complex blueprint. The colonial form of existence for certain organisms is perhaps a compromise between the two alternatives.
Diffusion off the left wall. In his book Full House, Steven Gould (1996) argues that the evolutionary record of increasing size and complexity is best explained not in terms of directional drive but as resulting from unpredictability, contingency, and accumulating variance. Thus, what could be called the phylogenetic ‘march of life’ might be viewed better as a ‘random walk of life’; it is visualized by Gould as emerging from an incipient form of minimal size and complexity, i.e., the primordial prokaryote, and diffusing away from that boundary. His metaphor is the ‘drunkard’s walk’ away from a bar (left wall): the person will sooner or later end up in the gutter simply due to random movement. All that is needed is an impenetrable limitation on the left (represented in biology by irreducible simplicity) and time.
There will be inevitable progression overall to the right, though this is purely from randomness conveying the appearance of directionality. Gould’s interpretation has received mixed support and has been variously modified, for example, by imposition of a ‘penetrable’ wall on the right (Knoll and Bambach 2000). The right wall would represent evolutionary limitations imposed by physiology or architecture that halt further diversification for an extended period. Examples of such right walls could include the evolution of hyperthermophily in certain bacteria, or the evolution of the eukaryotic cell, or multicellularity. Indeed, any of the evolutionary ‘major transitions’ in life postulated by Maynard Smith and Szathmary (1995), or the ‘megatrajectories’ proposed by Knoll and Bambach (2000) (Table 4.3), would qualify as right walls.
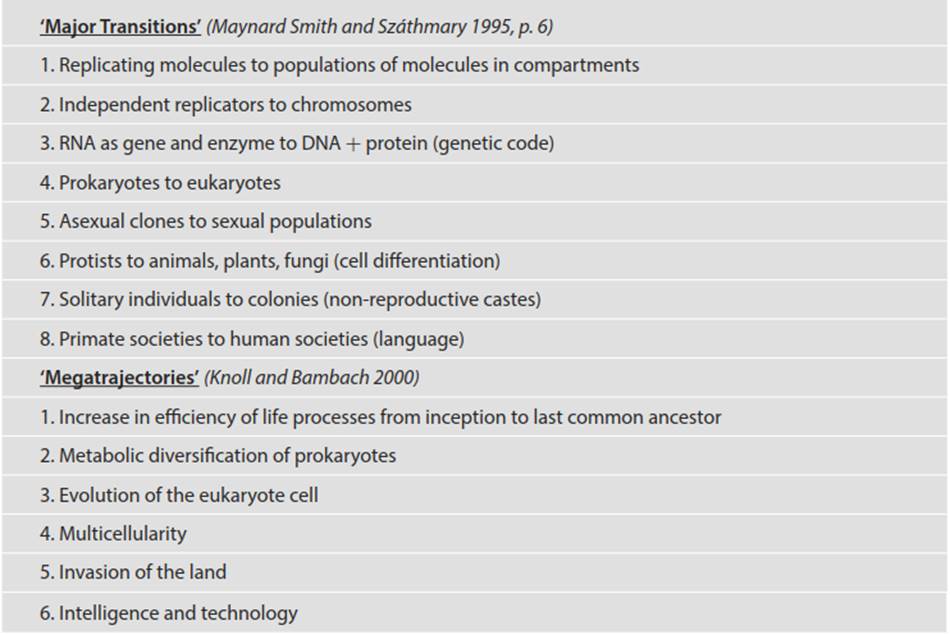
Table 4.3. Major evolutionary steps in the history of life
While, overall, biotic diversification within each of the ‘plateaus’ following an evolutionary breakthrough accords with an interpretation of increasing variance, the major transitions themselves in effect reset the evolutionary clock and impose a pattern of directionality within clades (Knoll and Bambach 2000; see also Vermeij 1999).
Date added: 2025-06-15; views: 210;
