Unitary and Modular Organisms: An Overview
Design of the Unitary Organism.Unitary organisms, represented by most mobile animals, have a standard number of appendages fixed early in ontogeny and follow sequential life cycle phases predictably. These are the sort of organisms that are the favorite subjects at zoos and for televised nature programs. Their growth is non-iterative, i.e., not based on a repeated multicellular unit of construction, and is typically determinate, i.e., of strictly limited duration. They display generalized or systemic senescence, and are generally unbranched. Reproductive value (a measure of current fecundity and anticipated future survival and fecundity; see Chap. 6) increases with age to some peak and then declines.
The most important evolutionary implication of a unitary design is that the genetic individual (genet of Chaps. 1 and 2) and the physiological individual are the same entity. The unitary organism begins only at the start of the life cycle from a single cell, typically a zygote.
Harper (1977, pp. 515-516) illustrated the direct correspondence between the genetic and numerical individual by observing that a count of rabbits measures the number of genotypes and also gives an estimate of biomass. Likewise, within a factor of about 10, it is possible to estimate roughly the number of individuals if the biomass is known. Because form is determinate in unitary organisms (as is cell number within relatively strict limits), appendages are fixed in number: you could determine the number of rabbits by counting the legs in a population and dividing by four or the ears and dividing by two.
Design of the Modular Organism.In contrast to unitary organisms, the zygote in modular organisms produces a basic multicellular unit of construction (module) that is iterated indefinitely, giving progressively more modules, which in aggregate constitute the genetic individual. Plants and sessile benthic invertebrates are classic examples of modular organisms. A typical, easily visualized module in the case of plants is the leaf together with its axillary bud. White (1979) captured its essence when he referred to “the plant as a metapopulation.” The number of modules— whether they are leaves or roots on a tree, hyphae of a fungal thallus, bacterial cells in a colony, or polyps on a coral—is indeterminate and the organism has extreme phenotypic plasticity. It is for this reason that a simple count of plants, unless their size is known, provides so much less information than a count of unitary animals (Harper 1977, pp. 25-27).
The major contrasting features between unitary and modular organisms are summarized in Table 5.1 and several modular organisms are shown in Fig. 5.1 (Harper et al. 1986; Hughes 2005). The inherently modular physical characteristics of fungi and bacteria are apparent when compared with the modular macroorganisms in this simplified figure. As will be developed in the following sections, whether an organism is unitary and mobile, or modular and sessile, probably has as great or greater biological significance than its size (microorganism vs. macroorganism).
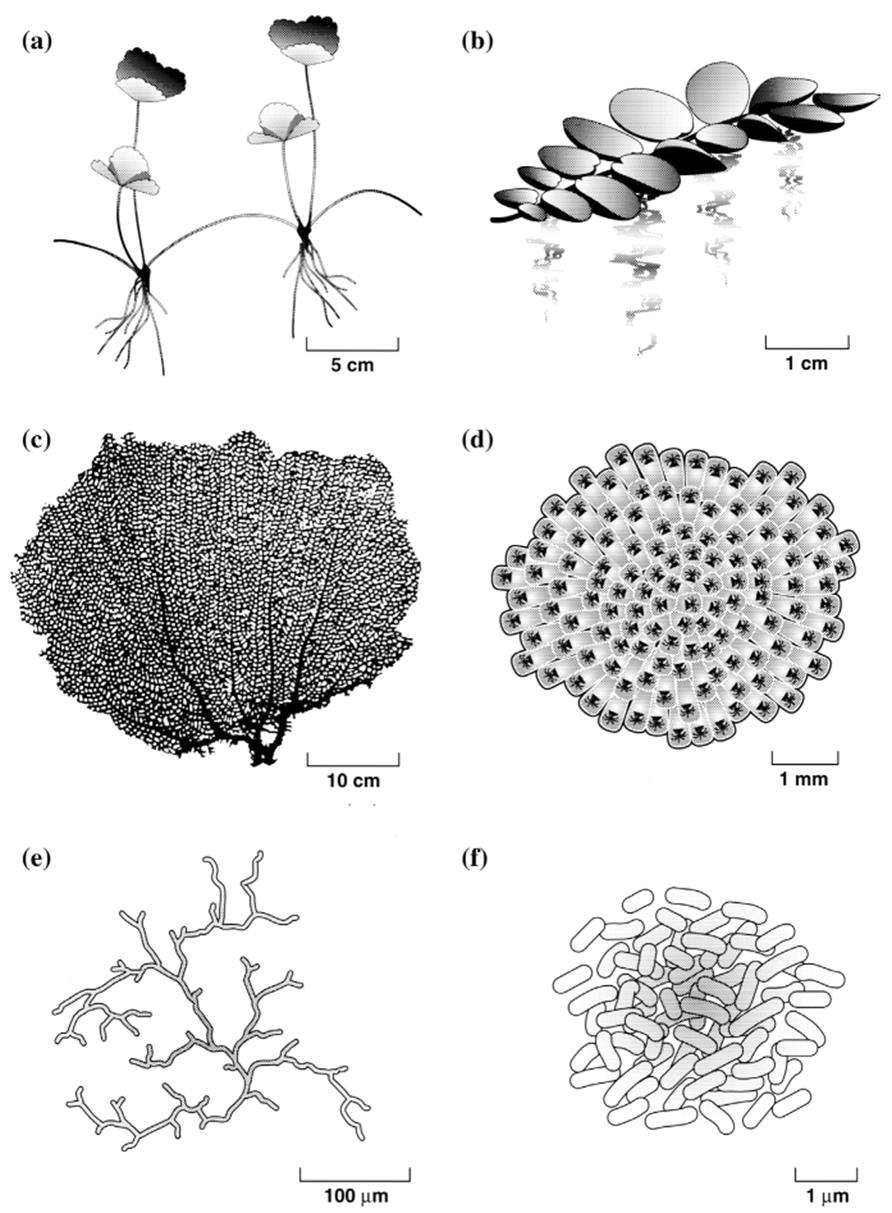
Fig. 5.1. Some modular microorganisms and macroorganisms. a A clonal terrestrial plant (strawberry) showing two ramets; compare with Fig. 5.2; b a clonal, floating aquatic plant (Salvinia); c a sea fan coral (Gorgonia); d a colonial bryozoan (Membranipora); e mycelium of a fungus; f microcolony of bacteria. Note the modular architecture of the fungus and bacterium
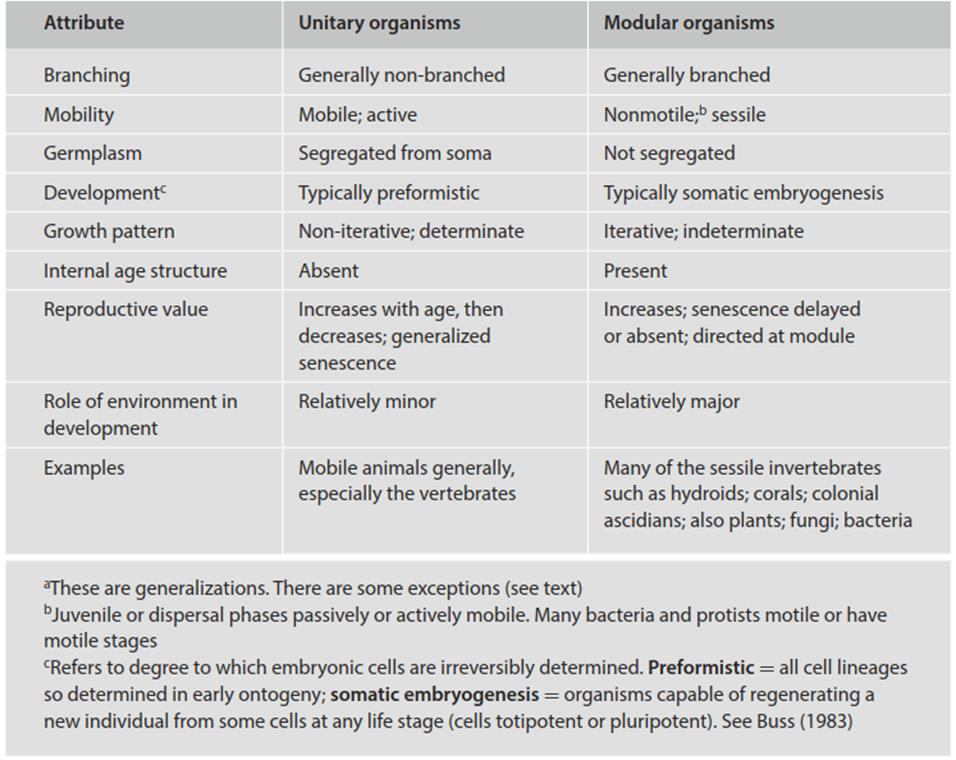
Table 5.1. Major attributes of unitary and modular organismsa (Andrews 1994)
Modules may remain attached and contribute substantially to the organism’s architecture. This is the case for trees and corals, much of which often consist of accumulated dead modules. Alternatively, in the case of some organisms, modules either individually or in clumps can operate as physiological individuals, i.e., ramets, at a level of organization that they are or can be functional on their own when detached (strawberry or the creeping buttercup, Ranunculus repens), or naturally budded or sloughed off from the parent as part of the developmental program (lichens; corals; the floating aquatic plant Lemna).
The ramets in aggregate constitute the genetic individual or genet; in other words, the genotype is fragmented (Fig. 5.2 and Harper and Bell 1979). Thus, modular organisms frequently grow clonally, that is, by the formation of individuals descending entirely from a common ancestor and thus of nearly identical genetic composition (though subject to genetic change over time; recall clonal discussion in Chap. 2 and see Jackson et al. 1985; Hughes 1989; Monro and Poore 2009b). In such cases the ramets are typically the ‘countable units’ in a pasture of white clover, Trifolium repens, or that arise from plating a bacterial suspension culture onto an agar medium in a petri dish. The pasture would consist of multiple clones of white clover, each represented by many individual ramets, which are the ‘plants’ or ‘plantlets’ of white clover. Likewise, the petri dish would consist of a clone of the bacterium represented by dozens of ramets or bacterial colonies, each in principle having arisen from a single cell (see below and later discussion of bacteria and fungi).
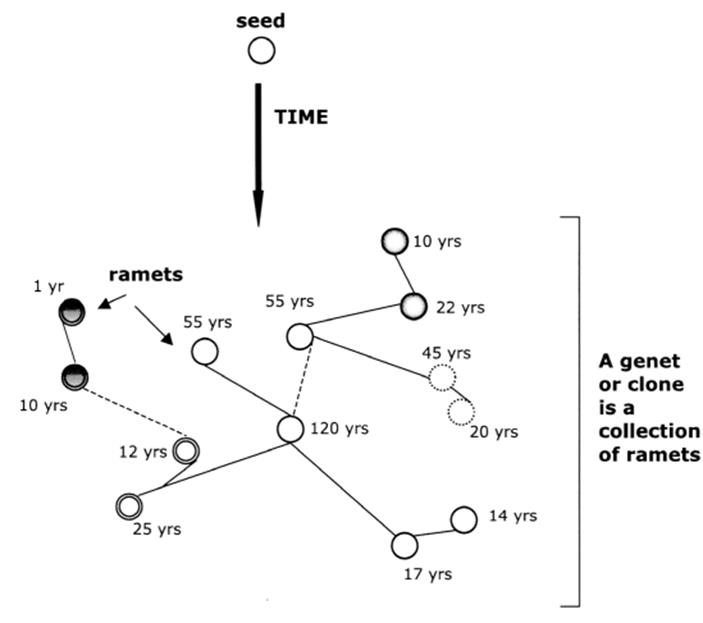
Fig. 5.2. Clonal growth through time originating from a zygote (seed) whose genotype sets the basic ancestral lineage of the clone. Ramets (circles with numerical age designations) are functionally independent members of the genet or clone and are born and die at different times. Initially, the ramets are effectively genetically identical though they diverge over time due to accumulating somatic mutations and the clone becomes a genetic mosaic, represented here by the different degrees of shading (see Chap. 2 and especially Fig. 2.8). Though functionally capable of independent existence, ramets may remain attached (solid lines) permanently, or degrade or break (dashed lines) depending on the species and conditions. Each ramet in this case is represented by an individual aspen tree in a grove that collectively is the genet. From Ally et al. (2008). Reproduced from Molecular Ecology by permission of John Wiley and Sons, ©2008
Perhaps, the most eloquently stated impression of a clone is that by Janzen (1977b) who viewed a dandelion population as one that “contains a small number of highly divided Els [evolutionary individuals] with very long lives and very low population growth rates and which exist through the harvest of a highly predictable resource (p. 586) ... the El dandelion is a very large tree with no investment in trunk, major branches, or perennial roots. It has a highly diffuse crown’ (p. 587) ... [note that although the dandelion plant has flowers and forms seeds, many populations of dandelion are exclusively asexual, a product of apomixis; Chap. 2].
Before taking up the functional and evolutionary implications of modularity, a brief detour into terminology and definitions is necessary because of the very similar and in some cases overlapping attributes of modularity, clonality, and colonial growth habits. Although some ecologists use the terms clonal and modular interchangeably (Jackson and Coates 1986; West et al. 2011), modular growth is more appropriately regarded as being in some ways analogous to but distinct from clonal growth. Asexuality and clones were discussed in some detail in Chap. 2. Organisms that reproduce asexually are clonal and often, but not invariably, modular.
Conversely, plants are clearly modular but only some grow clonally. And, there are some clonal unitary creatures. Examples include mobile animals that reproduce parthenogenetically or apomictically, such as aphids, rotifers, lizards, and earthworms. Finally, clonal organisms may be non-colonial (solitary, often dispersed over large areas) or colonial in growth habit.
The term colony is used broadly here to mean the tendency of units related by descent to clump (see zoological terminology in Jackson et al. 1985; Hughes 1989, 2005; West et al. 2011). These units, which are modules or groups of modules in the case of modular organisms, may be physically linked together (as in rhizomes and the basal or root suckers of many clonal plants) or not linked (clumps of Lemna; bacterial cells or colonies; aphids), though closely associated. Sponges remain a difficulty as they are really neither modular nor colonial. They can, however, reproduce asexually and so are clonal. Many of the attributes of clonality overlap with those of modularity (Table 5.1; Sackville Hamilton et al. 1987; Hughes 1989, 2005). The unitary/modular dichotomy can be viewed as primarily a morphological separation, whereas demarcation based on a solitary/colonial habit is physiologically informative; and the aclonal/clonal distinction carries demographic implications (for historical review of terminology and life history insights, see Grosberg and Patterson 1989).
Implicit in the above remarks is that, generally speaking, what constitutes an individual for modular, clonal, or colonial organisms cannot be sharply defined. Predictably, this ambiguity has spawned considerable debate and sematic difficulties going back at least to the 1970s (Boardman et al. 1973; van Valen 1978; Larwood and Rosen 1979). While proceedings from major symposia in the 1980s on clonal organisms (Jackson et al. 1985) and modular organisms (Harper 1986; Harper et al. 1986), and subsequent debate (Hughes 2005; West et al. 2011) provide thought-provoking perspectives, the gulf between approaches and terminology of the botanists and zoologists is quite evident. The historical confusion exists primarily because of two levels of complication. First, for clonal macro organisms, the physiologically functional individual does not correspond to the genetic individual. The ratio of genets:ramets is 1:many. The modular discussion above was focused on plants as an example, but applies equally to clonal invertebrates (see, e.g., Sanchez et al. 2004; Hughes 2005). Among corals, logical arguments could be made depending on a particular context for the individual to be represented by the polyp, by numerous polyps together forming a colony, by the zooid, or even by an entire reef (reviewed by Rosen 1986).
Second, the genetically mosaic nature of bacterial and many fungal individuals confounds strict boundaries. In these microbes, the genet itself is frequently discontinuous: The organism through its life cycle may be in a state of genetic flux, comprising both many ramets and frequently more than one genet as the original clonal lineage (especially for bacteria) becomes genetically diffuse due to somatic mutation and other more extreme genetic changes such as horizontal gene transmission (bacteria) or heterokaryosis (fungi; see below and Chap. 2). Occasionally, genetic change occurs in more regular fashion as in a balanced dikaryosis in some fungi (Chap. 2) or an event in a complex life cycle (Chap. 6). These ‘complications’ need to be taken into account in considering their evolutionary biology and the extent to which they are inherently modular.
Date added: 2025-06-15; views: 356;
