Phalanx and guerrilla microorganisms. Branching networks
Phalanx and guerrilla microorganisms. Clearly the descriptors phalanx and guerrilla also fit the growth patterns of other sessile, clonal organisms. Indeed, the specific epithets of some animals (e.g., the corals Pectinia paeonia, Pavona cactus) are derived from architecturally similar plants (Harper et al. 1986). Crustose (mat-forming) lichens grow as phalanxes as do some corals, bryozoans, and clonal ascidians; other corals and the foliose (aerially branched) bryozoans and lichens have, relatively speaking, a guerrilla-type habit (Harper 1981a, 1985). Marine benthic invertebrates and algae are shown in Fig. 5.7. Of the six basic forms of sessile marine animals (Jackson 1979; see also Taylor and Wilson 2003), the ‘runners’ and ‘vines’ are essentially a guerrilla habit, while the ‘trees’, ‘plates’, ‘mounds’, and ‘sheets’ are to varying degrees phalanx in form (for terminology and architectural variation, see Ferrell 2008; Cherry Vogt et al. 2011).
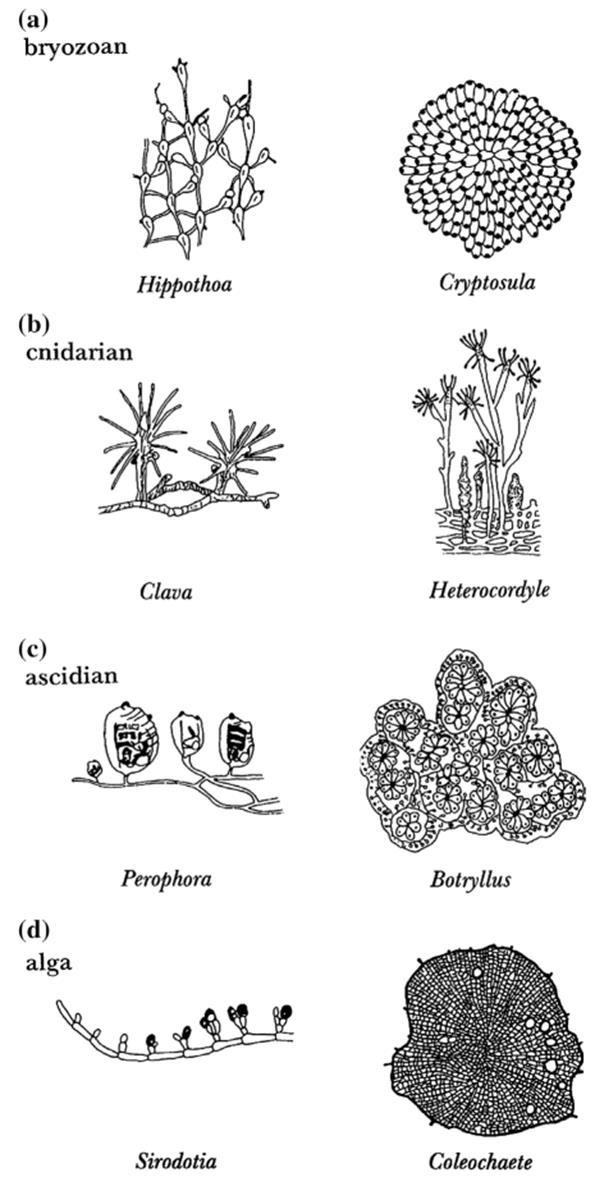
Fig. 5.7. Genera of sessilemarine invertebrates (a-c) and algae (d) representing the loosely spaced, 'guerrilla'(left column) and tightly packed, 'phalanx' (rightcolumn) life forms. From Buss and Blackstone (1991). Reproduced from Philosophical Transactions of The Royal Society of London B by permission of The Royal Society, ©1991
The above nomenclature from studies on invertebrates and plants is clearly relevant also to certain microbial patterns. Because of the plasticity of microbial growth, the terms can be applied in two contexts. First, as with other modular organisms, the descriptors can be used in a relative sense to compare different taxa. For example, Kohli et al. (1991; see also Kohn 1995) contrasted the clonal, guerrilla-type growth and colonization pattern of the fungus Sclerotinia sclerotiorum with the phalanx characteristics of Armillaria bulbosa. Other fungi and fungal-like organisms, particularly the Zygomycetes and Oomycetes, extend rapidly in guerrilla-like fashion. This is probably facilitated by their coenocytic nature, which permits unimpeded cytoplasmic flow and rapid communication (at the cost to the thallus of vulnerability to damage). Rhizopus stolonifer, as its name implies, colonizes territory rapidly by stolon-like runners that develop rhizoids and tufts of sporangiophores at ‘nodes’ (Fig. 5.6 noted earlier).
It is the creeping buttercup of the microbial world. At the other extreme, some fungi such as the apple scab pathogen Venturia inaequalis expand as a compact, scab-like mass, whether across a standard laboratory medium or in their natural substrata. Possibly the dimorphic fungi (which can alternate between a yeast and a hyphal phase) have the best of both worlds. Saccharomyces cerevisiae, for example, produces single cells in classical yeast-like fashion by budding, but under conditions of nutrient depletion forms filaments (pseudohy- phae). The pseudohyphal pattern has been interpreted adaptively as a means to enhance foraging, i.e., in the current context, to escape from RDZs (Gimeno et al. 1992; see also Andrews 1995). This example and the case of dimorphic fungi in general are discussed with respect to phenotypic plasticity in Chap. 7.
Second, the terms guerrilla and phalanx can also describe different growth phases of a particular organism (Andrews 1991). Basidiomycetes colonizing woody substrata in soil, or on the forest floor, commonly do so in exploratory, guerrilla-like fashion by producing elongated mycelial cord or branching rhizomorph systems (Thompson and Rayner 1982; Thompson 1984; Rayner et al. 1985; Boddy et al. 2009). When the fungus is within a nutrient cache a diffuse mycelial network tends to become established. This is strikingly similar to tropical lianas that initially produce rapidly growing ‘searcher’ shoots to find a suitable environment, which is then colonized by densely branching shoots (Strong and Ray 1975).
Branching networks. Plants and fungi share many attributes as sessile, branching, modular organisms, but some differences exist in their processes of resource capture. In part this is because, for the former, some resources move to the organism. Sunlight impinges on leaves (although there is a race to avoid becoming shaded by one’s neighbors), carbon dioxide and oxygen move by convective flow and diffusion, and inorganic nutrients tend to move quickly by bulk flow driven by transpiration. In contrast, fungi typically move to their resource, both by linear growth and dissemination. Here, dispersal by asexual spores moves the clone to new, potentially uncolonized resource patches. The arrangement of leaves, stems, and roots tends to be relatively well organized and structured relative to hyphal ramification (as noted above; also see Chap. 7), which is much more plastic and involves mutual cooperation. It is in this sense of communal effort that the fungi are like the social insects in resource capture division of labor, and genetic interactions (Rayner and Franks 1987).
Horn (1971, 2000) interprets the geometry of trees and the distribution and shape of leaves largely in terms of resource acquisition. His 1971 synthesis provides an explanation for why succession starts with trees intolerant of shade and proceeds to more tolerant species, rather than simply starting with the latter. The concept is based on calculations showing that net photosynthesis of leaves increases with light intensity up to about 20% of full sunlight. Beyond this, photosynthesis is not enhanced by further increases in light. Hence, strategies for light interception will be important whenever there is sufficient shade (in effect RDZs imposed by foliage) to reduce light to less than this 20% threshold value. Leaf placement in crowns of plants was viewed as either monolayered—leaves being concentrated at the periphery with few gaps and little overlap—or multilayered—leaves being randomly scattered vertically and horizontally throughout the canopy.
Where light is abundant as in the open fields typical of early successional environments, the multilayer was considered to be adaptive. In shaded, late successional environments, the monolayer form can theoretically grow faster. This is because the multilayer can produce several tiers of leaves in the open habitat without reducing intensity on the lowermost to less than the critical 20% level. In the shade, however, photosynthetic gains of the lowermost tier may not compensate for respiratory losses, so the advantage would swing to the monolayer design.
In overview, the branching pattern of modular organisms, and especially those that are clonal, has generally been interpreted in the context of foraging for energy and nutrients. Most of the research has been done with plants because of their morphological diversity and accessibility and increasingly has involved complex modeling and computation (e.g., Wong et al. 2011; Campillo and Champagnat 2012). Architectures, represented in the extreme by guerrilla and phalanx forms, are usually assumed to be adaptive though this assumption has rarely been directly tested and the sparse evidence is mixed. Form could also result to varying degrees from, for example, a search for refuges or defense, and is inherently constrained by the principles of biomechanical design or possibly even shaped by a purely mechanical process akin to ‘self-organized criticality’ (the classic example of criticality is the ‘avalanches in a sand pile’ analogy—see Bak and Paczuski 1995; Sanchez et al. 2004).
The architecture of sessile marine animals (Jackson 1979; McKinney and Jackson 1989) and seaweeds (Koehl 1986; Martone et al. 2012), particularly those in the intertidal zone, has been interpreted relatively less with regard to optimal nutrient acquisition and more in terms of resistance to hydrodynamic forces, substrate stability and characteristics, boring organisms and predators, and promotion of juvenile recruitment. However, comparison of a food acquisition model with a space limitation model has shown the former to predict better than the latter the morphology, distribution, and abundance of bryozoans under most conditions tested (Okamura et al. 2001).
The shape of plants must also be a compromise to multiple demands, of which light interception is only one, though admittedly an important one. Trees, because of their size, have to withstand strong winds, translocate fluids in a long plumbing system, disperse seeds, and in some climates shed snow loads, as well as display leaves efficiently for light interception and gaseous exchange (see Chaps. 4 and 7 and Koch et al. 2004, 2015).
Many influences are covariant and correlations may be direct or inverse. For example, adaptations for shade tolerance may conflict with those for drought tolerance (Valladares and Niinemets 2008) and functions such as mechanical stability and translocation efficiency impose engineering constraints (Niklas 1994a; Vogel 2003; Read and Stokes 2006). To use the words and metaphor of Wright (1932) and Niklas (1994b), the genetic analysis of such complicated adaptations resembles fitness walks over very complex landscapes. These morphological trade-offs by sessile organisms to multiple demands nicely illustrate the point made in Chap. 1 that every phenotype is necessarily a compromise among different and often opposing selection pressures. Traits have no significance in isolation from the whole organism (Dobzhansky 1956). Natural selection acts on the aggregate of what is available; perfection in form or any other attribute is not necessary for reproductive success and indeed cannot be achieved.
Date added: 2025-06-15; views: 202;
