Longevity, 'Potential Immortality', and Somatic Mutation
Age-specific schedules, important in life tables for the population biology of unitary animals, cannot be applied directly to genets of modular organisms (for unitary/modular comparisons, see Chap. 4 in Begon et al. 1996). This is because the inherent variability in modular organisms means that age is not a strong predictor of probability of death, reproduction, or growth. However, modules have an age structure with associated physiological characteristics and life tables for various modules, such as leaves or lateral branches, have been constructed (Reich et al. 2004).
Particularly in clonal modular organisms, the number of reproductive units tends to increase exponentially with age and this exponential increase can continue indefinitely (Harper and Bell 1979; Watkinson and White 1985; Harper et al. 1986). As long as the birth rate of ramets exceeds their death rate, however, the genet not only persists but expands. This state of affairs has been called “potential immortality” and is taken up under Senescence in Chap. 6. If true, Hamilton’s (1966) postulate that senescence is an inevitable consequence of natural selection does not apply.
Worded differently with somewhat different emphases, this means that asexual multiplication not only increases the size of the genet, but spreads the risk of death of the entire genet (see later section, When Should a Clonal, Modular Organism Divide?). Another, perhaps unexpected, consequence of exponential growth is that where genetic individuals continually increase in fecundity, the frequency distribution of fecundities of genets tends to become log-normal, i.e., very few individuals contribute the great majority of the zygotes for the next generation (Harper 1977; Harper and Bell 1979).
Numerous direct (e.g., counting growth rings in trees) and indirect (e.g., measuring the area or diameter of clones) methods exist to determine the age of modular organisms (de Witte and Stocklin 2010), although most involve degrees of inference and assumptions and are subject to various sources of error. For example, determining the age of a clone based on its size assumes several things: that age and size are closely correlated; that the full extent of the clone can be accurately identified as the same genetic entity; and that the clone expands as a perfect circle with a linearly expanding radius (Ally et al. 2008). Molecular techniques, such as the accumulated divergence in microsatellites due to somatic mutations, are increasingly being used as ‘ontogenetic clocks’ to estimate the extent and age of clones. Mock et al. (2008) studied clones of trembling aspen (Populus tremuloides) in the western USA, while Ally et al. (2008) used 14 microsatellite loci in studies of the same species in southwestern Canada. Their results with respect to clone age/size are discussed later. While it is not simple to distinguish mutational changes from allelic variation, somatic mutation can be inferred presumptively when an individual ramet differs by only one allele at one locus but is otherwise identical to the preponderant clonal genotype. These along with test procedures and caveats are discussed well both by Ally et al. (2008) and Mock et al. (2008); for broader considerations, see Arnaud-Haond et al. (2007).
Large and extremely long-lived genets have been recorded, subject to the sources of error noted above, particularly among clonal plants (Table 5.2): an immense clonal patch ofquaking aspen (Populus tremuloides) has been estimated at 10,000+ years and to weigh on the order of 6 x 106 kg (Kemperman and Barnes 1976; Grant et al. 1992; Ally et al. 2008; however, see Mock et al. 2008); creosote bush (Larrea tridentata) in the Mojave Desert of California at approximately 11,000 years (Vasek 1980); and the rare Tasmanian shrub Lomatia tasmanica at 43,600 + years (Lynch et al. 1998). Watkinson and White (1985) suggest that among clonal plants the main causes of death of old genets include fire, disease, and competition.
Dormancy, coupled with various forms of asexual multiplication and dispersal, would all act to reduce the risk of death to entire genets. Fungal clones can also be sizeable and long-lived. Dickman and Cook (1989) found several genets of the wood-rotting fungus Phellinus [=Poria] weirii estimated to be older than 1,000 years in mountain hemlock (Tsuga mertensiana) forests of Oregon. The fungus spreads outward from a focus of infection primarily by root-to-root contact. Sibling ramets of the various fungal genets survive forest fires that periodically destroy large areas of the stands. The facultative tree-root pathogen Armillaria bulbosa, which radiates through soil by cord-like rhizomorphs, is ascribed as being among the largest (occupying at least 15 ha and weighing in excess of 10,000 kg) and oldest (at least 1,500 years) organisms (Smith et al. 1992).
The issue of somatic genetic variation was introduced earlier (Chap. 2). Some evolutionary biologists have downplayed its importance either by arguing that all somatic cells, including variants, ultimately arise from a proximal common ancestral source (the zygote) and therefore are not very different (pp. 244-247 in Maynard Smith and Szathmary 1995), or by questioning whether such variants are evolutionary meaningful (appreciably heritable) (Harper 1988). As noted in Chap. 2, the evolutionary importance of somatic variation depends on the ontogenetic program of the individual and ranges from being likely relatively negligible (organisms with preformistic development) to potentially significant (cases of somatic embryogenesis).
If the mutated cells are destined to remain somatic (not transmitted to offspring), as is typical in the former case, then the changes of course are effectively evolutionary dead-ends. However, when the mutation confers a difference in the ability to survive and compete among other cell lineages during development, and become subject to either cell lineage (germ line) selection or gametic selection, it is potentially important (Otto and Hastings 1998). Cell lineage selection may lead to offspring through gametes or via various asexual processes such as fragmentation or sporulation. Gametic selection alludes to competition among gametes for fertilization and is probably significant in plants, which have a gametophyte generation (Chap. 6). (Gametes in higher animals, in contrast, are typically a transient phase and express the diploid genotype of their progenitor cells; thus they do not vary as extensively phenotypically and are not subject to as extreme selection pressure; Otto and Hastings 1998.)
Most individuals are genetic mosaics because most are composed of great numbers of cells and genes, which are subject to mutations usually caused by damage over time or by replication errors (Gill et al. 1995; Otto and Hastings 1998). Examples of the kinds of mutation were discussed in Chap. 2, but broadly speaking they include any change to the genome of a cell that is transmitted to the progeny of that cell. Rates in general per gene per individual generation have been given as 10-7 to 10-4, or as 10-5 to 10-4 per individual generation for mitotic recombination in plants (reviewed by Otto and Orive 1995; Otto and Hastings 1998; an ‘individual generation’ is generally taken to mean from a parent single-cell stage to an equivalent offspring single cell, i.e., from zygote to zygote). The impact of a mutation within a mosaic individual depends on its nature and the degree to which it accumulates, i.e., the number of cell divisions per individual generation. These vary with the organism (e.g., 50 such divisions occur in maize from zygote to zygote; reviewed by Otto and Hastings 1998). If the mutation is beneficial, germline selection can substantially increase its occurrence among the individual’s reproductive cells, and hence its chance of ultimately being fixed in the population. At the level of the clone, variation can influence fitness (Monro and Poore 2004, 2009a) and potentially be of adaptive value (Folse and Roughgarden 2011).
Whitham and Slobodchikoff (1981) apparently were the first to postulate that as a result of somatic mutation trees are genetic mosaics. Significantly, they argued further that the somatic heterogeneity presented to insect pests may enable plants to counteract, on a relatively short-time scale not otherwise possible, the adaptations of their herbivores to overcome usual systemic resistance conferred by routine meiotic recombination. What came to be known as the ‘genetic mosaicism hypothesis’ was later extended to clonal animals (Gill et al. 1995). Somatic mutation is especially germane to clonal plants because such organisms: (i) are frequently very large (many somatic cells); (ii) are frequently long-lived, (mutations can potentially accumulate; however, see below); and (iii) do not have a segregated germ line, so mutations arising somatically can be transmitted to offspring, both sexually and asexually. The tissues of importance with respect to somatic mutation in plants are the meristems because they are, as the name implies, effectively the reservoir for the stem cells of plants (Weigel and Jurgens 2002; Heidstra and Sabatini 2014).
The specific type of meristem associated with extension of the plant axis, and also typically the production of ramets, is the apical meristem of shoots and roots. The organization of such tissues and implications for the spread of somatic mutants will be briefly described in passing as an example of the difficulty in making explicit predictions of the consequences of somatic mutation in large, potentially long-lived organisms. In multiple papers, Klekowski and colleagues (e.g., Klekowski et al. 1985; Klekowski 2003) have shown how organization of the plant apical meristem influences the mutational process, with diplontic selection occurring among cell lineages in the meristem, and deleterious mutations accumulating within the individual genet potentially leading to ‘mutational meltdown’ (described below).
The most primitive group of vascular plants, the pteridophytes (ferns, horsetails, and lycopods), have a single, permanent apical cell. When this cell divides into two daughter cells, one remains as the meristem initial and the other initiates the lineage that differentiates ultimately into tissues of the plant body and, in some cases, ramets. Thus, if the initial mutates, the mutation (if nonlethal) will be passed on to the daughters and their clonal derivatives. Klekowski (2003) views the displacement of a mutant apical initial by adjoining wild-type cells as unlikely. This means that diplontic selection is not strong and the clone is prone to meltdown. Limited evidence, especially for ferns, supports this view (Klekowski 2003).
The second group of vascular plants comprises the gymnosperms (e.g., cycads, ginko, gnetophytes, conifers). Though the structural pattern varies among the groups of gymnosperms, the unifying meristematic feature is one involving multiple cells and of cytological zonation based on planes of cell division and activity, i.e., lack of a permanent apical initial. This pattern implies that diplontic selection is more likely to occur than in the pteridophytes and hence the impact of mutation to be less overall.
The third and most complex cellular arrangement applies mainly to the angiosperms (flowering plants), which typically have two clonally distinct meristematic tissue zones in the apical meristem: the tunica, consisting of one or more peripheral layers of cells; and the corpus, a mass of cells overlain by the tunica. The tunica is involved primarily in surface expansion, whereas the corpus contributes volume to the growing shoot (Chap. 5 in Esau 1965; Chap. 6 in Evert 2006). Because the pattern of cell division differs in the two areas, somewhat isolated subpopulations of meristematic cells develop. Although diplontic selection is maximized within the subpopulations, a mutant that arises in one layer can be sustained by the wild-type cells in a different layer nearby, so stable chimeras may form and persist (Klekowski 2003).
So, to what extent are somatic mutations accumulating in clonal populations and what are the consequences? Lynch et al. (1993) describe the phenomenon of mutational meltdown attributed to genetic drift in small populations where natural selection cannot purge slightly deleterious mutations, which accumulate and reduce fitness. This has been documented in small populations (Zeyl et al. 2001) of yeast asexually propagated under laboratory conditions. The consequence of mutations to bacterial clones was discussed in Chap. 2. In clonal plants, random drift may overwhelm diplontic selection between mutated and wild-type cells in an apical meristem and eventually lead to the fixation of a somatic mutation in some or all of the ramet shoot apices of a particular genet. This is clear from theory summarized above and is also evident from natural observations and controlled experimentation (e.g., Gill et al. 1995; Klekowski et al. 1996). Plant anatomy and ontogeny evidently exert considerable influence on the likelihood of this outcome.
The genetic study of trembling aspen alluded to earlier included analysis of the immense (>40 ha) ‘Pando’ clone in central Utah (Mock et al. 2008; DeWoody et al. 2008). Remarkably, in the 256 ramet samples from this genet, only six variants of the predominant multi-locus genotype were detected and all were single-step mutations. This suggests remarkable homogeneity and possibly relatively young age notwithstanding earlier reports of great longevity of aspen and other clonal plants (Table 5.2). The boundaries of the clone detected genetically were, remarkably, almost identical to those detected earlier by morphological methods (Kem- perman and Barnes 1976). An additional 40 genotypes were distributed along the periphery of ‘Pando’. Unlike the single-step variants, these differed at 4-7 alleles, and it is unclear whether these are of somatic or sexual origin (DeWoody et al. 2008).
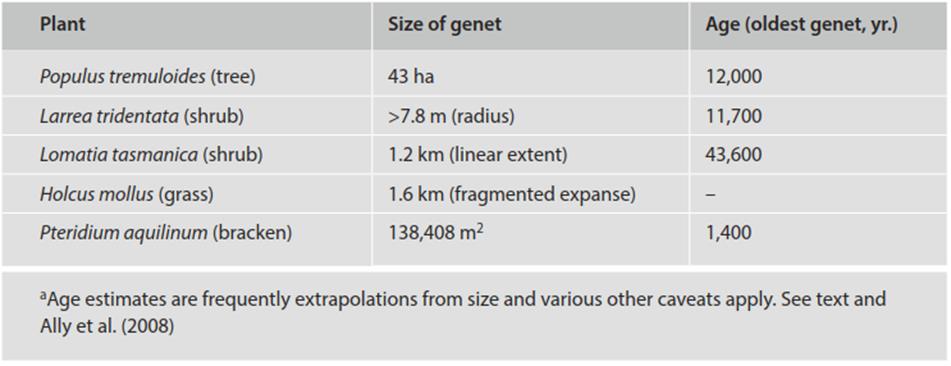
Table 5.2. Estimated size and longevitya of some clonal plants (abbreviated from de Witte and Stöcklin 2010 and references therein)
Peripheral patches of genetically distinct clones were also detected in the Canadian study (Ally et al. 2008). The Canadian study also documented reduced male fertility (decline in average number of viable pollen grains per catkin per ramet) with clone age. This was attributed as probably being due to somatic mutations that accumulate over time affecting sexual fitness only, with little to no effect on clonal growth (Ally et al. 2010). The results are reviewed and the interesting implications discussed in Chap. 6 under Senescence.
Date added: 2025-06-15; views: 210;
