Occupation of Space and Capture of Resources
As noted at the outset, modular organisms in general have a fundamentally iterative, branched structure. Most occupy relatively fixed positions and by growing capture space and thereby other resources. Branching networks can be characterized based on certain general properties (Gross and Sayana 2009) and in the living world have been studied extensively among both plants and animals (Stevens 1974; Harper 1985; Waller and Steingraeber 1985; Sanchez et al. 2004). One attribute, for example, is the degree to which the modular subunits are consolidated and the extent of interaction among subunits of the same and different individuals. Of particular interest is how the organization of subunits affects the efficiency of nutrient and energy capture by the overall genet. For plants, resources may take the form of light entering the canopy of a tree, or mineral nutrients in the rhizosphere; for a bryozoan, they are food particles in the surrounding water column; for a fungus, the resource may be a fallen tree.
For the modular sessile organism, modules act to capture resources, which sooner or later tend to become locally exhausted creating a resource depletion zone (RDZ). By overtopping rivals, the tree canopy of a superior competitor creates in effect a RDZ of light in the understory. Roots forage for and are plastic enough to respond to nutrient patches (Ruffel et al. 2011; Chen et al. 2016) in a manner analogous to the aerial portions of plants responding to light or filamentous fungal hyphae responding to resources in the leaf litter or soil. In the case of the rhizosphere (Fig. 5.4), the size of a RDZ, and if such a zone develops at all, depends largely on whether a particular solute is absorbed relatively faster than water (for dynamics of nutrient acquisition by roots, see Chapman et al. 2012). If it is, then the concentration at the root (module) surface will be lowered. In contrast, if water is taken up faster, the solute
may accumulate at the root surface and diffuse away. (It should also be remembered that root zones can actually be areas of enrichment for certain nutrients, such as amino acids and carbohydrates, which are exuded by the host and are thereby ‘hotspots’ for microbial activity.) Thus, the onset and dimensions of RDZs depend, among other things, on how rapidly resources are used by the module and resupplied by the medium.
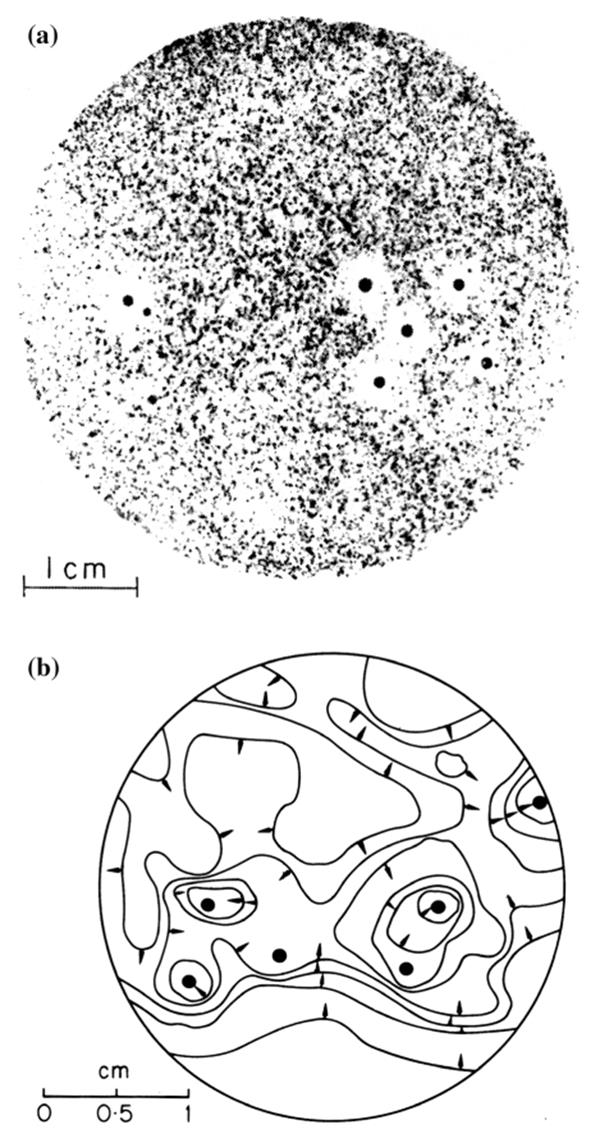
Fig. 5.4. (Top) Resource depletion zones appearing as halos around onion roots (black dots) in cross section in an autoradiograph of soil labeled with 35SO4. (bottom) The concentration profile of 35SO4 shown as contours of equivalent concentration based on densitometer tracings. Both figures from Sanders (1971); courtesy of F.E.T. Sanders, University of Leeds
The search process. There has been considerable theoretical discussion of optimal growth pattern and search strategies to capture resources (see broader, conceptual discussion in Chap. 3 and Hein et al. 2016). Here we focus mainly on the challenge for a sessile organism in acquiring resources, which involves modulating growth so as to strike a morphological compromise between optimal search and exploitation. In other words, the evolutionary goal in principle is to efficiently locate resources while minimizing overlap of RDZs. As noted in Chap. 3, how this is accomplished is as much a part of optimal foraging theory, broadly construed, as are strategies related to the active pursuit of prey.
Most mobile, unitary animals actively search their environment in organized fashion using their neural/motor circuitry (e.g., by processing olfactory, visual, acoustic, and other cues); modular organisms tend to do so by positioning their modules at feeding sites. Clonal plants, for instance, may accomplish this by alternating feeding locations (e.g., leaves and roots at horizontal nodes) with spacers (rootless stems; non-photosynthetic tissue) (Bell 1984). Plant shape is drastically affected both above- and below-ground by sunlight distribution/intensity and soil nutrient patches, respectively (de Kroon et al. 2005; Chen et al. 2016).
Bell visualizes four basic feeding behaviors among organisms: (i) a single (unitary) organism, mobile along some sort of search or foraging route (animal); (ii) a single, immobile organism feeding at one site (aclonal plant); (iii) a clonal organism feeding at many sites reached by growth of a branching system that potentially fragments; and (iv) a ‘community’ of the same organism foraging as a unit along a defined branching structure, each feeder contributing to the organized whole. Bell uses in this last case as examples social insects such as the army ant, although slime molds and similar microorganisms would be the microbial analog (Fig. 5.5).
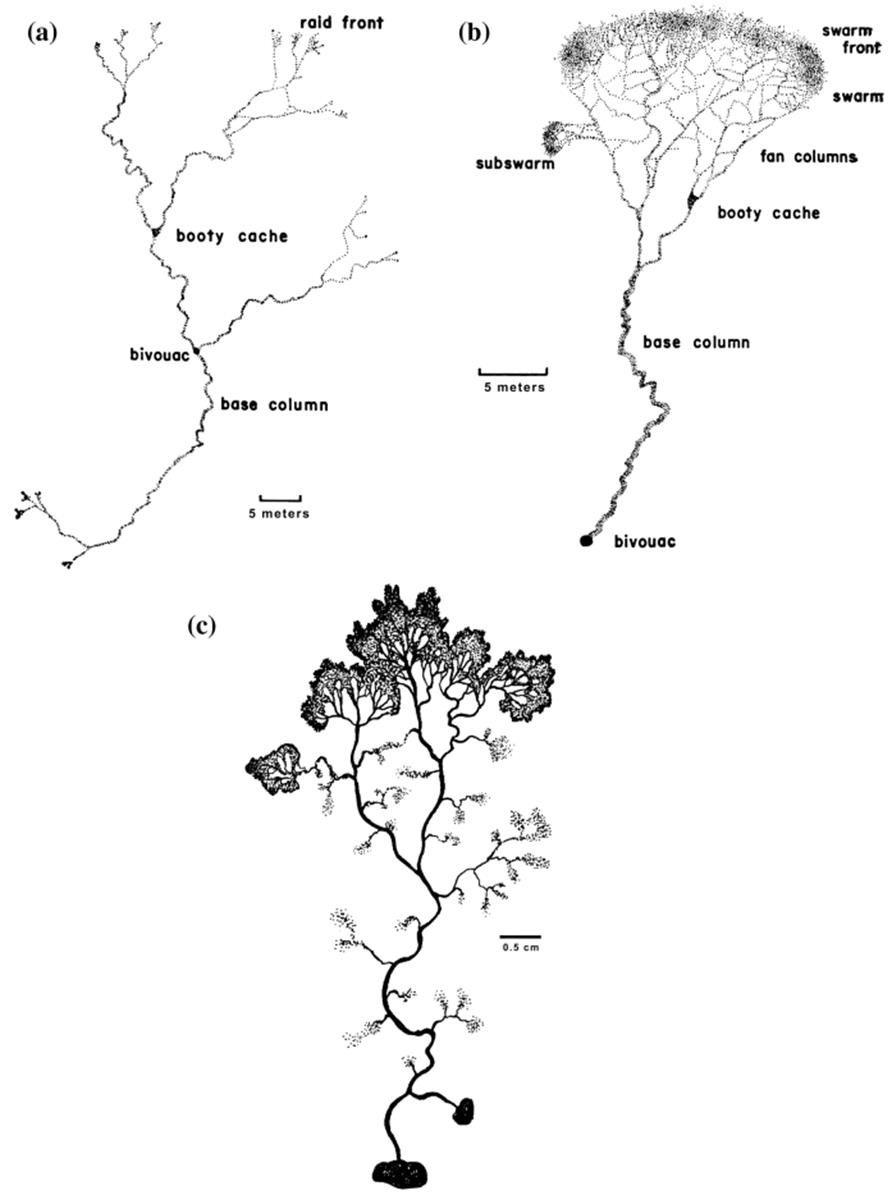
Fig. 5.5. Similarity in architecture of some growth network systems. a, b Raiding patterns of two species of army ants. The scale bars in A and B are 5 m. From Rettenmeyer (1963); reproduced by permission of The University of Kansas Science Bulletin, ©1963. c Foraging pattern of the slime mold Physarum polycephalum. The scale bar is 0.5 cm. Drawn from a photograph of a plate culture, courtesy of Tom Volk, University of Wisconsin-La Crosse
Assuming some information is available to the forager on resource distribution and the foraging objective at one extreme is to leave no resource site unexplored, a spiral growth pattern without branching is theoretically the most economical of effort (Chaps. 2 and 4 in Stevens 1974; Harper 1985; Bartumeus et al. 2005). This is analogous to an efficient search strategy for locating an object such as a sunken ship when something is known about its presumptive location. Fossil evidence shows that the primitive sea-floor slug Dictyodora (a unitary, not a modular organism) fed in a spiral pattern—a foraging behavior that allowed it to cover the whole of an area of sediment systematically (Seilacher 1967; Sims et al. 2014). Similarly, a species of ginger, Alpinia speciosa, has a hexagonal branching pattern with characteristics of a tight spiral (Bell 1979).
Simulations suggest that a spiral search also can result from random (Levy) walks if certain conditions are imposed such as cueing or avoidance of selftrails (Chap. 3 and Sims et al. 2014). This is more efficient than a simple ‘scribble’ pattern, but it covers an area less efficiently than tight ‘meandering’ because the spaces between the spirals are not exploited. In order of appearance among various worms, snails, and trilobites, scribbling appeared about 600 Mya and was replaced by a meandering and occasionally a spiraling mode between 425 and 500 Mya. Complex meanders and dense double spirals, considered the most efficient foraging patterns, did not appear until the Mesozoic (135-63 Mya) (Seilacher 1967; see also Seilacher et al. 2005).
At the other extreme, to exploit a pocket of an unlimited resource with minimal overlap, a linear growth pattern is best. The closest approximation to avoiding overlap while leaving no resource zone untapped is to branch and to change the branching pattern as growth proceeds (Harper 1985). Using a simple two-dimensional, hexagonal array of equi-spaced dots, Stevens (1974: Chap. 2) compared four basic patterns—the spiral, meander, explosion (center dot connected directly to each outlying dot), and various forms of branching—in terms of four geometric attributes: uniformity, space-filling, overall length, and directness. Each pattern has advantages.
However, if the organism must both search and exploit with limited biomass at its disposal, branching, which is both short and direct, is the best compromise. (Similar patterns result in a three-dimensional array but it is noteworthy that the spiral becomes a corkscrew or helix and cannot completely fill space, unlike its two-dimensional counterpart.)
In interpreting theoretically optimal search strategies, the issue of compromises to multiple demands needs to be kept in mind (see Chaps. 1, 3, and see below). Perhaps key amongst these is that there are other forces shaping growth pattern than simply the search for and extraction of resources. And, with respect specifically to plants and resources, although at any one instant only one resource may be the limiting factor, in reality many resources will be limiting over time. Since RDZs are dynamic and vary with the resource, a strategy that may be optimal for capturing a particular resource (e.g., readily diffusible nitrate ions in soil) may not be necessarily so for another (e.g., weakly diffusible phosphate ions in soil).
There are other considerations. The complex spatial pattern of a clonal plant in a field will reflect to varying degrees the search for light, water, soil nutrients and, especially, the positive and negative effects of neighbors (and neighbors here may be of three types: a different species; the same species but a different clone or genet; or the same clone but a different portion or member). Indeed, a detailed study over many years reaching conclusions emphasizing the first-order importance of neighbors was reported by Harper and colleagues in a Welsh pasture (see e.g., Turkington and Harper 1979a,b).
Date added: 2025-06-15; views: 186;
