The Evolution of Self Recognition: Fusion, Conflict, and Defense in Modular Organisms
Because modular organisms are sessile and grow in indeterminate fashion, often by asexual increase as clones or colonies, they tend to contact parts of themselves and parts of other organisms in their immediate vicinity. On one hand, this situation presents the hazard of overgrowth or displacement by a competitor, or invasion by a foreign cell line (or pathogen). In other words, one can view such interactions purely in terms of two competitive pressures: at the level of the individual (colony) or at the level of the cell line (Buss 1990; Laird et al. 2005).
On the other hand, the fusion of compatible conspecific genotypes offers potentially substantial advantages such as immediate increase in size. Another is the complementation of physiological repertoire between partners or even the emergence of novel traits. Such attributes have adaptive value in a contest for limited space as often occurs on the surface of, or within, a localized habitable substratum (by marine encrusting invertebrates or fungal hyphae, respectively). Thus, one would expect there to be strong selection pressure for the ability to determine self from non-self and that this discrimination likely would have arisen fairly early in geological time. Indeed, recognition ability transcends the issue of unitary/ modular design and seems to be a correlate of multicellularity: some manifestation of self/ non-self recognition occurs in essentially all multicellular organisms across all phyla. There are even suggestions of analogous recognition processes among some extant social bacteria that operate as collectives (Gibbs et al. 2008).
Here we will focus on issues directly relevant to modular micro- and macroorganisms and on allorecognition phenomena (operative among members of the same species) rather than xenorecognition (among members of different species). Incidentally, sessility implies that the effects of one neighbor on another can be specifically documented, whereas interactions among mobile (unitary) organisms usually have to be assigned abstractly to ‘density effects’ (Harper 1981a). Most of the attention has focused on genetic interactions between individuals, though even the most primitive modular organisms can respond to physical, chemical, and mechanical cues (Chap. 7 and Mydlarz et al. 2006). The formation of fusion chimeras has been most extensively documented in the fungi and the sedentary clonal benthic invertebrates, including sponges, bryozoans, cnidarians, and ascidians. Both groups (see following examples) share a mode of development known as somatic embryogenesis, i.e., they do not sequester a distinct germline and a given cell lineage can contribute to both somatic and germ cells throughout development (Buss 1987; see Sidebar and also Table 5.1 and comments in Chap. 2).
Colonial invertebrates When two colonies of a benthic invertebrate fuse, stem cells can move throughout the chimera with the result that one partner can more-or-less overwhelm the other, thus becoming disproportionately represented in sexual or asexual propagules. In extreme forms of takeover the result is ‘somatic cell parasitism’ by the aggressor and genomic replacement of the victim (Buss 1982, 1987). Chimeras have been observed in detail in the field and laboratory (Stoner and Weissman 1996) and, in the case of Botryllus (below), invasion by relatively few stem cells is enough to accomplish a chimera (Laird et al. 2005).
As discussed below and in Chap. 2, an analogous fusion/rejection interaction occurs in the fungi, although it is the nuclei rather than stem cells that move within the chimeric hyphal network, which in fungal semantics is generally referred to as a heterokaryon (Roper et al. 2011, 2013). In both situations there would be significant selection pressure to limit fusion to genetically compatible partners. Genetic surveillance systems oversee such interactions and are coupled to defensive response systems of varying sophistication that counteract an invader.
The genetic basis for allorecognition in colonial invertebrates has been studied for the most part in the ascidian Botryllus (Phylum Chordata) and the hydroid Hydractinia (Phylum Cnidaria). In both cases, at least one polymorphic fusibility/histocompatibility locus controls the outcome, where fusion depends on the sharing of one or two alleles (Grosberg 1988; Magor et al. 1999; Cadavid 2004; Rosengarten and Nicotra 2011). Studies on Botryl- lus show that randomly paired isolates rarely fuse and it is inferred that the fusibility locus is highly polymorphic with more than 100 alleles. However, Grosberg and Quinn (1986) showed that localized sites are settled by sibling larvae, which distinguish kin based on shared alleles at histocompatible loci.
This means that developing colonies fuse with each other or with parental colonies at much higher rates than by random association, so potential benefits accrue to both kin members (see also De Tomaso 2006). Hydractinia colonies, shown in the Sidebar, grow as a surface encrustation on gastropod shells inhabited by hermit crabs. Allorecognition responses have been documented in multiple studies over more than a century and take three forms: (i) fusion (establishment of a common gastrovascular system within a few hours), (ii) passive or active rejection (the latter involving a specialized organ and tissue destruction mediated by stinging cells), and (iii) transitory fusion (Cadavid 2004) (Fig. 5.9).
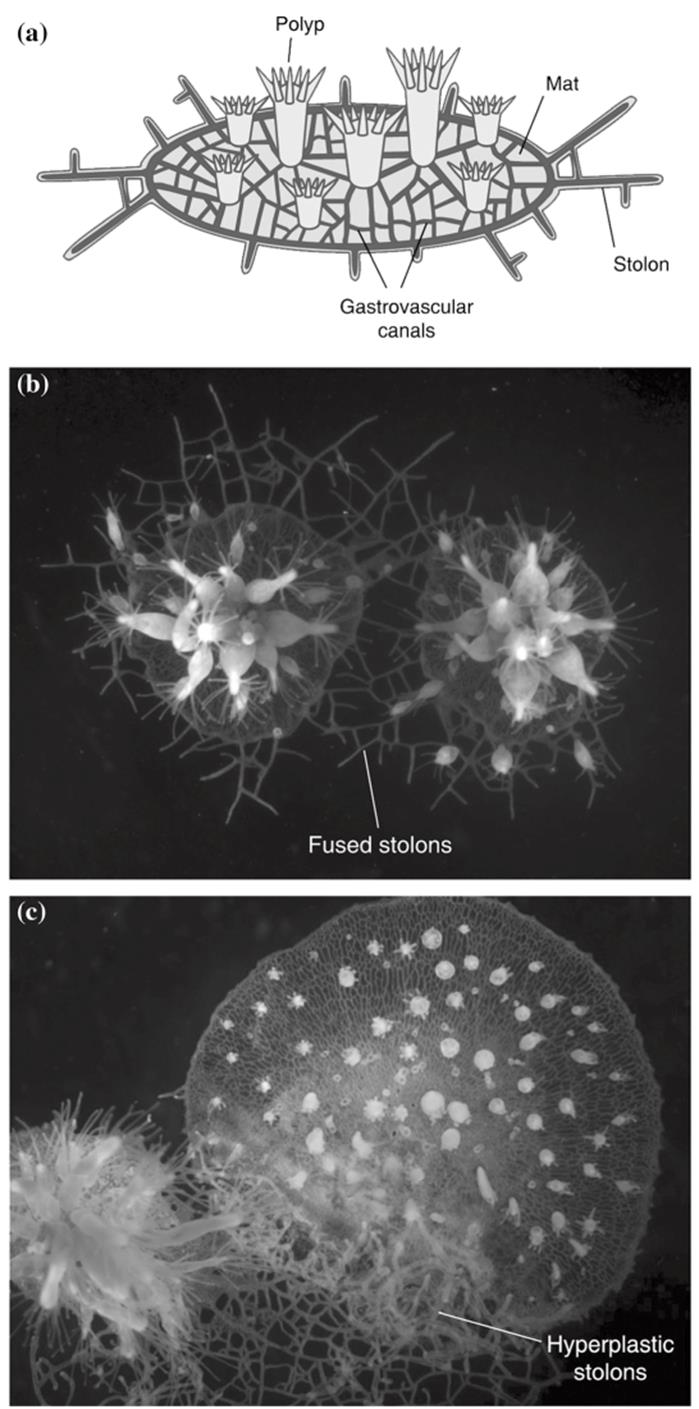
Fig. 5.9. Allorecognition patterns in the cnidarian Hydractinia symbiolongicarpus, a colonial hydroid. a A colony showing the main parts. b Fusion between two histocompatible colonies. Note fused, small, web-like stolons barely visible in background. c Rejection between two incompatible colonies. Note enlarged, 'hyperplastic' stolons, typical of an aggressive response. From Rosengarten and Nicotra (2011), reproduced from Current Biology by permission of Elsevier©2011. Figure (C) is from Poudyal et al. (2007); reproduced from Proceedings of the National Academy of Sciences by permission of the National Academy of Sciences, USA, ©2007
The Hydractinia allorecognition complex appears similar in some ways to Botryllus, but is believed to be more complex, at this point assumed to involve at least two linked loci (reviewed by Rosengarten and Nicotra 2011). Fungi Most fungi (primarily the ascomycetes and basidiomycetes) grow by an extensively radiating mycelium, noted earlier, that acquires nutrients from a patchy environment by ramifying upon, within, or at the interfaces between substrata, such as between soil and leaf litter (Andrews 1995; Bebber et al. 2007). Inevitably parts of the same and of different fungal individuals come into frequent contact. Intra-organism hyphal fusions are advantageous in terms of communication, homeostasis, transport, and repair.
The overall network geometry of branching and fusions appears to be developmentally programmed and designed to optimize the conflicting demands of foraging with cytoplasmic mixing and transport (Roper et al. 2013). Inter-organism fusions can be advantageous in several ways as addressed below. Whether individuals fuse and go on to form a chimera is regulated by various incompatibility systems, including (i) somatic or vegetative incompatibility; (ii) sexual incompatibility; and (iii) intersterility. Of these, the most intensively studied and broadly analogous to macroorganisms (in some cases probably homologous, see below) is somatic incompatibility (Chap. 2 and Worrall 1997).
Where fusion between different genotypes occurs (or in the case of somatic mutation), two or frequently more, genetically different nuclei occupy the same cytoplasm (heterokaryosis; Chap. 2). As noted in Chap. 2, the extent of heterokaryosis in nature and its ecological advantages have been debated going all the way back to the original studies in the 1940s and 1950s (Pontecorvo 1946; Jinks 1952). In general the benefits relate to increased phenotypic plasticity, enhanced physiological capability and, in the case of pathogenic fungi, possibly increased virulence. Buss (1987) argues further that the filamentous fungi, by virtue of their cytology, are uniquely predisposed to both benefits from chimerism, as well as to be vulnerable to invasion.
This sets up a situation where the different nuclei are potentially cooperators or competitors (a theme echoed by more recent investigators, e.g., Roper et al. 2011). Because of the porous, pipeline-like structure of hyphae, potentially thousands or even millions of genetically different nuclei can occupy the same interconnected network and travel quickly by bulk flow over relatively long distances (Roper et al. 2011, 2013). Many species, principally in the phyla Oomycota and Zygomycota, completely lack cross-walls (septa) so the cytoplasm is continuous, multinucleate (coenocytic), and the nuclei can readily move long distances relatively unimpeded (Roper et al. 2013). Species in the phyla Asco- mycota and Basidiomycota, and their associated asexual states, are generally septate. These septa vary structurally, are to varying degrees porous to the passage of nuclei, and in some cases can even discriminate even among different nuclei. This appears to be one form of control to protect the individual thallus from systemic takeover by deleterious mutant or invader nuclei.
Aside from somatic fusion events and mutation, heterokaryosis also arises in other ways, the principal one being mating. Unlike the case with other macroorganisms, karyogamy in the fungi frequently does not immediately follow plasmogamy. In the ascomycetes, mating- type heterokaryons usually are physically restricted to certain cells (ascogonium and ascoge- nous hyphae) and the dominant life cycle phase, barring fusions among different genotypes, is as a haploid homokaryon (Worrall 1997).
The success of hyphal anastomoses and heter- okaryon formation is regulated by heterokaryon incompatibility (het) loci that limit fusions to closely related homokaryons; if any of these typically multiple loci contain different alleles in the partners then fusion fails and lysis occurs. Increasing numbers of het genes are being sequenced; they encode various polypeptides, including mating-type transcriptional regulators (for elaboration on the genetics see Glass et al. 2000; Glass and Kaneko 2003). The mating-type locus can also function in some fungi as one of the somatic incompatibility loci (Worrall 1997).
In the basidiomycetes, typically the heterokaryon is the result of mating and is called a dikaryon (Chap. 2) because each hyphal compartment has a pair of nuclei, one donated by each partner or mating type. However, unlike the case with the ascomycetes, the genomes are very different at other loci (James et al. 2008). The allocation of the two nuclei per cell, their synchronous division, transcription, and even their mutual spacing are exquisitely balanced and regulated (Gladfelter and Berman 2009; also see discussion of clamp connections in Chap. 2). Enforced dikaryotization prior to karyogamy ensures nuclear balance, though where the asexual propagules are monokaryotic the two nuclei may compete for representation in the conidia.
The nuclear situation has led to two competing views on basidiomycete heterokaryons: either they operate functionally as diploids with selection operating at the level of the heterokaryon individual; or as populations of nuclei where the unit of selection is the individual nucleus (Rayner 1991; James et al. 2008; Roper et al. 2011). Though recent advances in cytological technique enable nuclear dynamics to be assessed precisely (Roper et al. 2013), the conceptual issue is essentially as framed about 70 years ago by Pontecorvo (1946). This returns us to the observations of Buss (1987, pp. 129-139), noted above, that the very structure of fungal hyphae that facilitates formation of genetic chimeras and resulting organism vigor renders the fungi vulnerable to aberrant (mutant) nuclei or nuclear invasion.
From this welter of details the important broader point emerges that these various fungal processes regulate self/non-self discrimination in a manner directly analogous to the colonial invertebrates. The systems have evolved to enable the ‘appropriate’ fusions and the associated ecological advantages to occur, while protecting the individual from somatic cell or nuclear parasitism (Buss 1982, 1987). Allorecognition and rejection phenomena apparently reflect convergent evolution in each of the major evolutionary groups, though it would be interesting to know whether they have arisen from a common ancestral gene(s) basal to the clades. As more genes are sequenced and the products identified, the picture will come into focus.
But the important and even broader message here is that recognition phenomena are fundamental and transcend the arbitrary cleavage of the living world into microorganism and macroorganism.
Date added: 2025-06-15; views: 188;
