Alternation of Generations
With the evolution of sexuality in eukaryotes, the regularized haploid and diploid phases that are morphologically striking in land plants and many algae (Niklas and Kutschera 2010) became dominant elements of the conventional life cycle. To vastly simplify, plasmogamy and karyogamy, typically involving gametes, establish the diploid phase, which is followed more or less rapidly by meiosis, restoring the haploid condition. However, karyogamy may not closely follow plasmogamy and there is a tremendous phylogenetic range in the number of mitotic cell generations separating the two events of meiosis and syngamy (Valero et al. 1992; Coelho et al. 2007): The fungi, for instance, are almost exclusively haploid (meiosis rapidly follows syngamy); the n and 2n phases are about equally prominent in many algae such as sea lettuces, Ulva spp. (meiosis and syngamy are separated in time and space); while in most animals the haploid condition is relatively brief, inconspicuous, and represented only by the gametes (syngamy rapidly follows meiosis).
Terminology varies, but generally speaking the three types of cycles are referred to as haploid, haploid/diploid, and diploid, respectively. As discussed in Chap. 2, some fungi are unique in having an extended dikaryotic (n + n) condition. Broadly speaking, the eukaryote life cycle is one in which growth and reproduction alternate with sexual fusion and meiosis; vegetative processes occur in the haploid phase, the diploid phase, or both. Growth during haploidy is a primitive condition, with diploid growth having evolved independently in numerous clades (Bell 1994). Representative organism life cycles and the nuclear states (n, n + n, 2n) of key basic stages are summarized in Table 6.1.
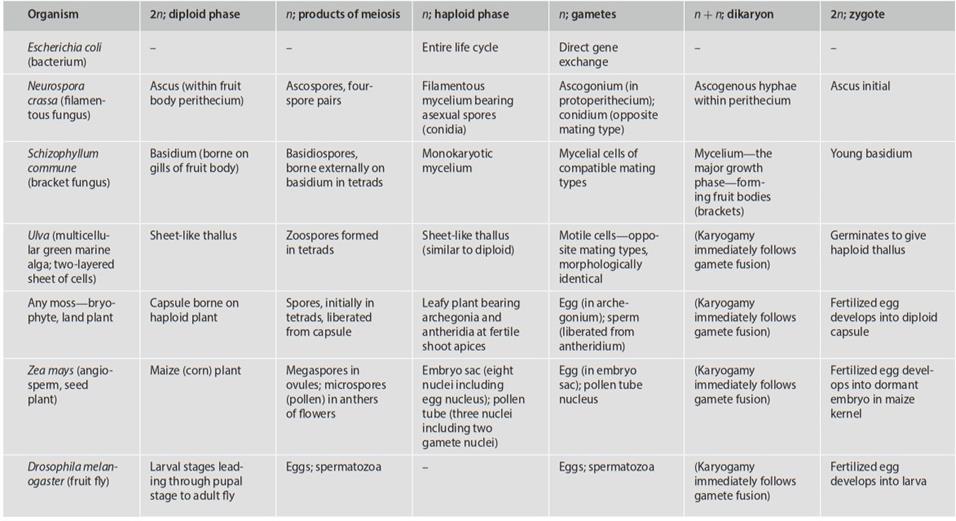
Table 6.1. The life cycles of some representative micro- and macroorganisms (modified from Fincham 1983)
In modern land plants (embryophytes) the life cycle is particularly remarkable (Walbot and Evans 2003; Niklas and Kutschera 2010). Here the products of meiosis are not gametes but spores that undergo mitosis to produce a haploid, multicellular body termed a gametophyte, which produces the eggs and sperm. The gametophyte alternates with a multicellular diploid sporophyte (spore-producing) generation that originates upon fusion of the gametes and ends with the production of haploid meiospores. All land plants (as well as some algae) display a conspicuous alternation of generations and the sporophyte is the dominant form among the more modern (vascular) lineages (tracheophytes: ferns, lycopods, horsetails, and seed plants). Among the higher seed plants (angiosperms), the nonphotosynthetic gametophyte typically is reduced to only 2-7 cells that rely on the sporophyte for nutrients. Conversely, among the earliest land plants (bryophytes: liverworts, hornworts, mosses) the gametophyte generation dominates, with the diploid phase characteristically being inconspicuous. These fundamentally different life cycles are called diplobiontic (alternation of two multicellular phases, one diploid and one haploid) and haplobiontic (only one multicellular generation), respectively (Fig. 6.3).
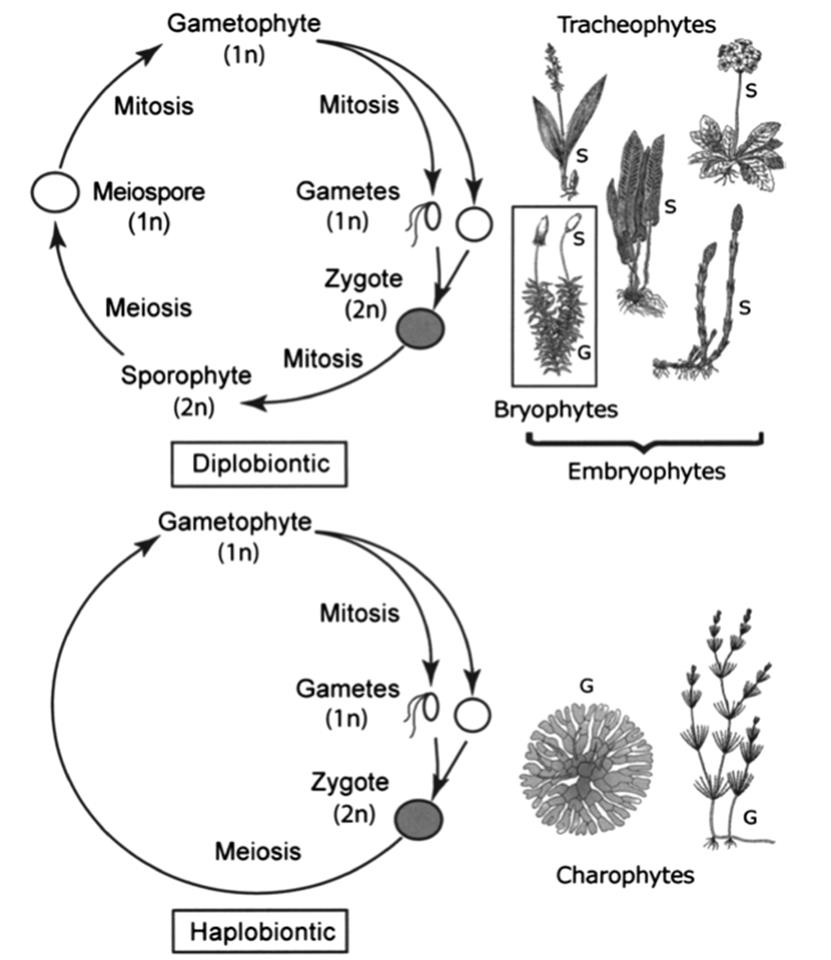
Fig. 6.3. Attributes of basic life cycles in the plant world. The modern land plants or embryophytes have a diplobiontic cycle (upper figure) where both the haploid gametophyte (G) and diploid sporophyte (S) are multicellular and more-or-less conspicuous. In contrast, the green algae, here represented by two charophytes (likely the group from which the land plants emerged), have a haplobiontic cycle (lower figure), where either only the haploid phase is multicellular (haplobiontic-haploid) as illustrated here, or only the diploid phase is multicellular (haplobiontic-diploid) as is the case for animals. See text for taxonomic nomenclature. From Niklas and Kutschera (2010). Reproduced from New Phytologist by permission of Karl Niklas and John Wiley and Sons, Inc. ©2009
Interestingly, the land plants evolved from the charophyte-like (stoneworts) ancestor within the green algae (see Chap. 4), i.e., from a condition where the gametophyte dominates and the single-celled diploid phase is only transient, to one where the sporophyte is ascendant (Niklas and Kutschera 2010; Evert and Eichhorn 2012). This ancient transformation probably resulted from a delay in meiosis possibly orchestrated by genes encoding regulatory homeoproteins that maintained sporophytic function and suppressed gametophytic development. A similar argument pertains to extant plants that undergo regular alternation of generations in their life cycles (Sakakibara et al. 2013). Indeed, Niklas and Kutschera (2010) suggest that the reproductive structures of the extant multicellular charophytes and embry- ophytes are homologous and that the embryophytes largely redeployed the ancient algal homeodomain gene network. An intriguing debate centers on whether the shift to a diplobiontic cycle was essential for transition from an aquatic to the terrestrial environment (i.e., a developmental constraint) or merely correlative (i.e., a phyletic legacy) with the successful colonization of land by plants (Friedman 2013).
Across the diversity of living organisms, life cycle patterns show an evolutionary trend to a preponderantly diploid condition, evidently a correlate of individuality and complexity. Numerous theoretical hypotheses based on genetic or ecological interpretations have been put forward in favor of diploidy (summarized by Hughes and Otto 1999; Coelho et al. 2007). In the main they relate to: (i) the masking of deleterious mutations by the normal allele; (ii) retention of the masked mutations as a genetic repository available for future use; (iii) the twofold greater amount of DNA enables new beneficial mutations to accumulate at a greater rate; (iv) the ‘additional’ allele can evolve new functions while the other copy is providing for ongoing function. However, the advantages of diploidy have been challenged and may be situation-specific (e.g., Zeyl et al. 2003); also, the trend to diploidy does not explain the maintenance of haploid and biphasic haploid/diploid cycles (Hughes and Otto 1999).
The green plant lineages beautifully illustrate the spectrum ranging from an essentially haploid gametophyte body, through a relatively balanced condition, to complete sporophyte dominance in the most advanced embryophyte phylum (for details and terminology see Graham 1993; Graham et al. 2009). By virtue of having the gametophyte of varying size and duration, plants can impose stringent selection during the genetically active haploid phase and likely have a lower genetic load than animals; this may be the reason that, unlike many animals, they do not have to designate a germ line early in development (Walbot and Evans 2003; however, see Szovenyi et al. 2013). Under some conditions, genes promoting haploid selection may be favored but not if such mutations are selected against in the diploid phase—a condition referred to as ‘ploidally antagonistic selection’ (Otto et al. 2015). The balance in such pressures and the broader genetic and ecological interactions governing biphasic cycles are explored by Rescan et al. (2016).
Date added: 2025-06-15; views: 203;
