Natural selection and the multicellular condition
The budding yeast Saccharomyces cere- visiae, well known in industry as well as in genetics and molecular biology, is preponderantly unicellular (Bruckner and Mosch 2012). Using a population of initially unicellular cells, Ratcliff et al. (2012) reenacted in contemporary time the evolutionary transition to multicellular- ity over geological time. They show in principle that at least the incipient stages can occur relatively quickly under appropriate selective conditions. Having arisen in the prehistoric microbiological world, why might multicellularity have been maintained by natural selection?
The main benefits attributed to increased size involve, broadly speaking, more effective resource acquisition or avoidance of size-selective predation (phago trophy). Additional advantages probably include (i) provision of storage reserves; (ii) provision of an internal environment protected by outer layer(s) of cells; (iii) allowance of novel metabolic innovations; (iv) enhancement of motility (Grosberg and Strathmann 2007). Bell and Koufopanou (1991) approach the question by defining the benefits of small size and then attributing the selection for large size to failure of the assumptions for benefit of smallness.
Their most simple theoretical case involves a life cycle of rapid growth from an initial to final size followed by fission. In a uniform, invariant environment with no interactions among individuals, fitness would approximate the growth rate r, with r approaching rmax in optimal conditions, being greatest for small organisms and decreasing with size (see comments on r-selection, later). Effectively this describes bacterial exponential growth dynamics in continuous culture. In more realistic cases where the environment varies in time and space and individual interactions occur, larger forms typically have several advantages.
The multicellular gliding bacterium Myxococcus is a nice example of how a consortium of cells can operate advantageously. Myxococcus secretes various enzymes that degrade other bacteria on which it feeds in its soil habitat. Likely the organism functions better as a collective than it would as individual foraging cells. In fact, it is social in its eating habits: as Kaiser (1986, p. 541) says, “Myxococcus feeds as a pack of microbial wolves, with each cell benefitting from the enzymes secreted by its mates”. Each cyst is a package of cells, ready upon germination for activity as a cooperative group, and the fruiting body is of sufficient size (~ 0.2 mm; Kaiser 2001) that it is readily transported by small animals in the soil. Such groups of cells and eventually multicellular assemblages are also more resistant to abiotic disturbance (erosion from surfaces, desiccation, UV injury, etc.) as is apparent from the biology of bacterial biofilms (Teschler et al. 2015).
An important consequence of the transition from unicellularity to differentiated multicel- lularity was the transfer in fitness from lower level units (cells or groups of cells) to the higher level individual (Buss 1987; Michod 2006, 2007; see also comments in Chap. 1). This arose inevitably as a compromise associated with cell specialization wherein formerly totipotent, independent cells gave up their autonomy to collaborate, thereby becoming subordinate and dependent on the group for survival and reproduction. This had some very important evolutionary consequences. Some cells acquired only a somatic role and could no longer form a new individual. Furthermore, the division of labor allowed fitness of the integrated group to be augmented over that of the average fitness of its component units.
Moreover, the origin of cooperation was followed by cheating, wherein a mutant cell lineage proliferates at the expense of the whole individual (Hammerschmidt et al. 2014). This situation arises by mutation in the original clonal lineage; an analogous threat arises when different genotypes fuse and one overtakes the other (Buss 1982, 1987; Rainey and De Monte 2014). Such genetic conflicts would tend to counteract the benefits of size increase (Grosberg and Strathmann 2007). That there are multiple defenses against such defections or invasion by other genotypes and that they are phylogenetically widespread, even into the most primitive of taxa, testifies to the threat that genomic conflict poses to the evolutionary individual.
These defenses include: passage through a single-cell stage typical of life histories, germ-line sequestration in many organisms, and various self/non-self recognition systems. Even in the earliest eukaryotes such countermeasures exist: In the cellular slime molds noted earlier, ‘the individual’ spends much of its life in disaggregated form as independently foraging cells on the forest floor and the individual reunites as a consolidated entity (and hence potentially vulnerable) for a relatively brief phase associated with reproduction; whereas in Volvox, defense takes the form of early differentiation of germ cells so that invasion by variants is limited to the brief phase of the first few cell generations early in morphogenesis (not unlike the embryogenesis pattern in many animals (see Buss 1987 and Chap. 5).
Ways to become large. Fig. 4.5 shows conceptually in simplistic terms the options for increase in size, with some examples from extant organisms. Up to a point, presumably set by physicochemical factors related to surface:volume exchange processes (section, Seeing the World, below), unicells can simply increase their volume. Indeed, as alluded to at the outset, a few species of prokaryotes are relatively immense, but generally unicellular eukaryotes are larger. Multicellularity confers the advantages of large size achieved by the building block approach. This method resolves metabolic concerns related to cell surface area:volume scaling by delimiting the size of the individual blocks, and allows for regularized genetic oversight of the developing soma by mitosis (Chap. 4 in Bonner 1988).
A developmental constraint here is the nature of the starting material: Walled forms of green algae developed extensive mul- ticellularity, whereas naked forms did not (Bell and Koufopanou 1991; Koufopanou 1994). A simple extension of the unicellular plan was for cells to divide but rather than separating be retained in one dimension (see OFig. 4.5). This results in unbranched filamentous growth. McShea (2001) describes insightfully for hierarchical organisms how clones and colonial organisms may have assembled and a possible geological timeframe for the events.
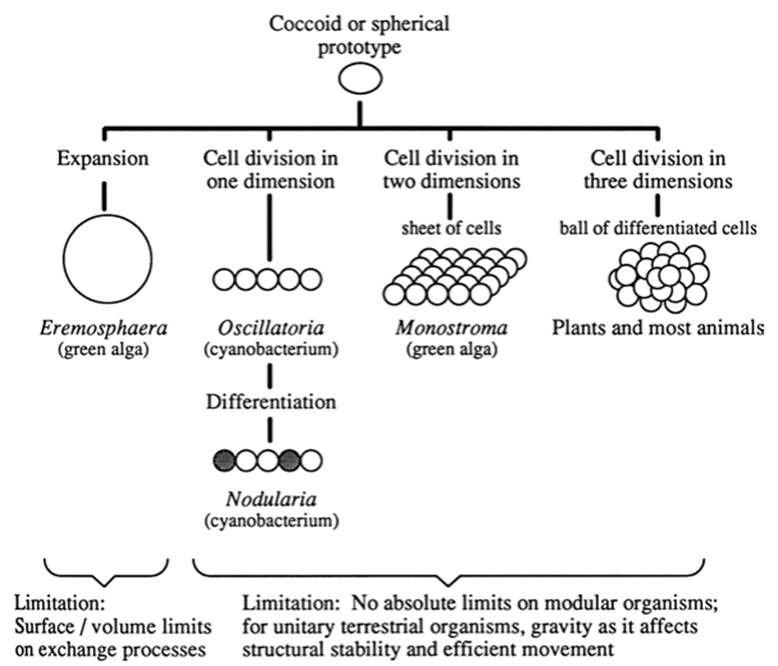
Fig. 4.5. Four simplified, hypothetical examples of how increase in size, multicellularity, and differentiation could have evolved. Spherical geometry is shown for simplicity; a very early shape also was filamentous. In addition to incipient multicellularity occurring from cohesion of the products of cell division, it could have resulted from aggregation of compatible cells. Representative genera are extant forms
The earliest multicellular eukaryotes were on the scale of millimeters and also took the form of linear or branched filaments (Carroll 2001). Presumably undifferentiated multicellular clusters (Pfeiffer and Bonhoeffer 2003) or filaments occurred first, followed by differentiated cells, such as heterocysts (specialized for nitrogen fixation) or resting spores known as akinetes in cyanobacteria (Rossetti et al. 2010; Flores and Herrero 2010; Schirrmeister et al. 2011). Because nitrogen fixation is an anaerobic process destroyed by even trace amounts of oxygen, unicellular cyanobacteria such as Gloeothece must alternate photosynthesis by day with nitrogen fixation at night. However, their differentiated, multicellular relatives such as Nostoc and Anabaena can simultaneously conduct photosynthesis in vegetative cells and fix nitrogen in the heterocysts.
There is some evidence that the multicellular species have a competitive advantage provided that day length is sufficiently long (Rossetti et al. 2010). A morphologically simple form of differentiated growth would have been to simply divide into two cells with segregated function. A contemporary example is the dimorphic (stalk cell, swarmer cell) stalked bacteria such as Caulobacter and Hyphomonas (this life cycle is discussed in Chap. 6; see also Brun and Janakiraman 2000). However, morphological simplicity of the stalked bacteria belies underlying developmental sophistication (Curtis and Brun 2010) and could not have been an early evolutionary event.
Butterfield (2009) reviews the pre-Edicarian (i.e., prior to 635 Mya) forms of multicellular organization, which included various colonial forms, unbranched or branched filaments, and monostromatic sheets. The latter arose from growth in two dimensions (represented by the extant green alga Monostroma in Fig. 4.5). The number and orientation of the planes of cell division, along with their location, largely set the few basic body plans in plants (Niklas and Kutschera 2009). These developmental processes have tended to be conserved over great evolutionary time scales. The primordial traces of the metazoan lineage are obscured and the incipient morphologies difficult to delineate from algae or other animate or inanimate structures in ancient fossils (Butterfield 2009; Marshall and Valentine 2010). However, many primitive as well as extant animals, particularly marine benthic species, are colonial (Raff 1996).
Colonies typically arise by the simple iteration of a basic unit, but also can result (as can other body forms) from the aggregation of compatible cells, tissues, or nuclei (Grosberg and Strathmann 2007; Bonner 1998, 2009). An aggregative phase is a regular component of the life cycles of several microorganisms, including myxobacteria, certain flagellates, and all slime molds (but especially the cellular slime molds), noted above. An extension of this aggregative style occurs in fusion chimeras, where different clones (usually of sessile organisms such as benthic invertebrates or terrestrial fungi) meet fortuitously and, if genetically compatible, merge (Buss 1982; Grosberg 1988). Thus, in conceptual and developmental terms, multicellularity can arise in two ways: either by clonal processes (repeated division of an original cell or zygote) or by aggregative processes. Bonner (1998) argues that the inception of multicellular- ity in aquatic lineages occurred when the products of cell division failed to separate, whereas in most terrestrial lineages multicellularity originated by the aggregation of cells or of nuclei in a multinucleate syncytium.
The eukaryotic cell as a pivotal development. Evolution of the eukaryotic cell was a major event in the development of macro organisms. The details of how this novel cell type arose remain unclear and there are no known intermediate types in the fossil record. Although the differences between prokaryotic and eukaryotic cells are many, the most significant overall advance was probably the compartmentation of the eukaryotic cytoplasm that allowed localization of cellular activities, together with an oxidative metabolism (in common with some prokaryotes) that improved energy extraction beyond that obtainable by fermentation. These activities were fostered by acquisition of endosymbionts capable of photosynthesis and respiration. Eukaryotic cells are on average 1000-fold larger in volume than prokaryotic cells. Even a unicellular eukaryote carries more DNA and in general more structural and genetic information than does a prokaryote. A stunning array of morphological forms is apparent even among the protozoa and single-celled algae.
The eukaryote design must have been a good building block for multicellular architecture, for it seems more than coincidental that the cells of all the more complex organisms are eukaryotic. Apart from establishing a lineage (the unicellular protists) that could obtain food by engulfing it, the primitive eukaryotic cell type gave rise to three fundamentally diverse body types: (i) multicellular, soft-celled, heterotrophic forms that digested food internally (primitive invertebrates); (ii) multicellular, hard-walled forms that digested food externally (the fungi); and to (iii) multicellular forms that, by symbiosis with bacteria, came to manufacture their own food (green algae and the higher plants). Prokaryotes approach this multicellular, differentiated condition in the complex structures of some of the contemporary fruiting myxobacteria such as Chondromyces (Fig. 4.6; close relative of Myxococcus noted earlier).
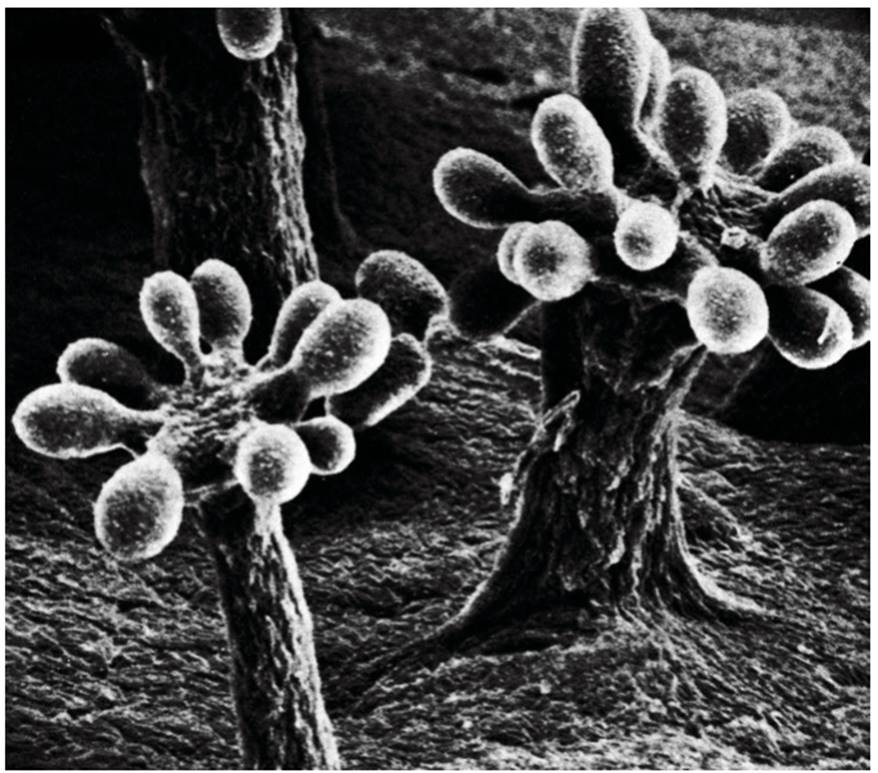
Fig. 4.6. Differentiation and elegant forms of rudimentary multicellularity among the bacteria: Mature fruiting bodies of the myxobacterium Chrondromyces crocatus showing elevated multiple sporangia (cysts), each of which contains resting cells embedded in slime. Photo courtesy of Patricia Grilione, San Jose State Universit
Nevertheless, the multicellular route for bacteria was little more than a detour and ultimately an evolutionary dead end. The eukaryotic avenue led to architecturally elegant forms of cell differentiation and the radiation of life. One of the earliest and most important developments was probably a boundary layer (the epithelium) separating the interior of an organism from the exterior and, in the case of plants and fungi, a complex and rigid but dynamic cell wall (Domozych and Domozych 2014).
The culmination in cell specialization is evident in the exquisite architecture and division of labor among the some 200 cell types of a vertebrate. The driving force behind this series of innovative events was that first the larger cell, then the multicellular macroorganism, by virtue of their size, were able to exploit some circumstances (environments, resources) better than or at least differently from the microbe. The new forms, through growth and activity, shaped their surroundings and in turn evolved in ways that their progenitors could not.
Date added: 2025-06-15; views: 246;
