Multicellular origins and the volvocine algal paradigm
The green alga Volvox and its relatives (three families and about 50 species) provide a fascinating case study in the transition from unicellularity to multicellularity and extensive differentiation, including many examples of inferred multiple origins and reversals (Herron and Michod 2008; Herron et al. 2009; Arakaki et al. 2013) in this monophyletic assemblage of lineages. All the volvocine algae are motile, haploid, facultatively sexual, photosynthetic eukaryotes typically inhabiting ponds or the calmer waters of lakes. Relative to the major phyla alluded to above, where the unicellular to multicellular transition is much more ancient (on the order of 1 Gya), these algae evolved relatively recently (~200-300 million years ago = Mya).
The forms spanned single- celled ancestors probably like present-day Chlamydomonas or Vitreochlamys to multicellular species varying from a few up to ~50,000 cells in the relatively complex, differentiated Volvox (Herron et al. 2009) (Fig. 4.4). The colonial forms range from relatively simple, totipotent, undifferentiated clumps of cells that remain attached after division (such as Gonium or Eudorina), to small colonies of various shapes (Pandorina), to a large sphere composed of dozens of cells with incipient differentiation in cell size and function (Eudorina, Pleodorina), culminating in thousands of biflagellate, peripheral somatic cells surrounding a few internal germ cells embedded in transparent, glycoprotein-rich extracellular matrix (Volvox noted above) (Kirk 2005). However, the intermediate gradations in organization are best interpreted not as a linear progression of transitional states but as alternative stable states (Larson et al. 1992).
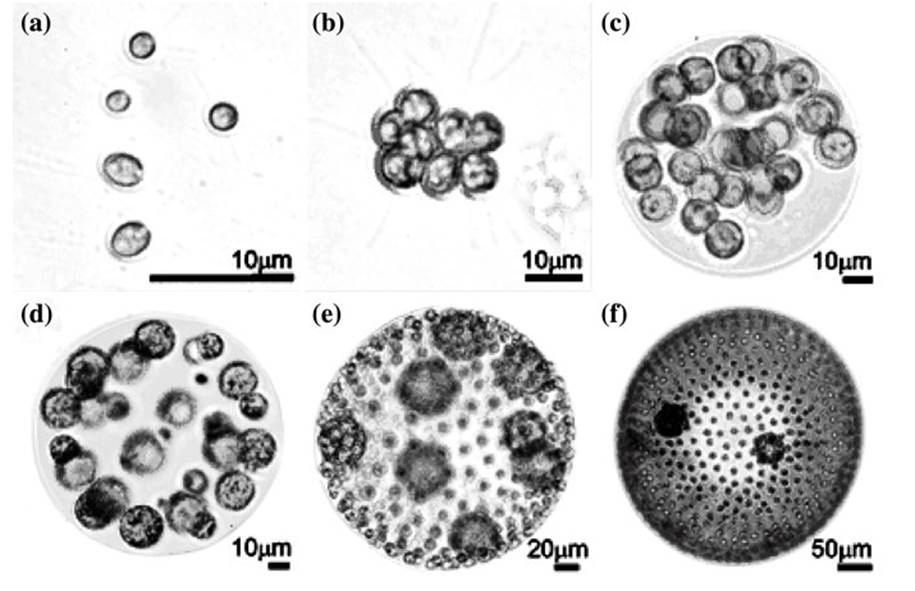
Fig. 4.4. The range of complexity within the volvocine green algae as represented by various unicellular and multicellular members: a Chlamydomonas reinhardtii, unicellular; b Gonium pectorale, a sheet of 8–32 undifferentiated cells; c Eudorina elegans, a colony of 16–64 cells, undifferentiated; d Pleodorina californica; e Volvox carteri; f Volvox aureus. The colonies in (d), (e), and (f) are differentiated into somatic (smaller) and reproductive (larger) cells. From Michod (2007); reproduced from Proceedings of the National Academy of Sciences ©2007 by permission of the National Academy of Sciences, USA
Along with increase in cell number in the volvocine lineage have come increased integration, intercellular communication, germ/soma specialization (division of labor), complexity, and individuality, as previously individual cells or small groups of cells coalesced to become higher order individuals (see comments in Chap. 1 and Michod 2007; Herron and Michod 2008). Genomic comparative analysis of the unicellular Chlamydomonas reinhardtii and its multicellular relative Volvox carteri shows that, with few exceptions, the increase in developmental complexity evidently centered on expansion of lineage-specific proteins rather than extensive protein-coding innovation (for analogous comparison with animals, see King et al. 2008). In other words, it did not involve major change in the original protein-coding repertoire, suggesting that ancestral genes were co-opted into new processes (Prochnik et al. 2010).
The evolution of germ and soma in the volvocaceans illustrates nicely the general principle of increasing complexity associated with multicellularity and the trade-offs inherent in cell specialization and associated division of labor. Because the algal cells are denser than water they need motility to maintain an optimal position in the photic zone; extensive day/night migrations occur, presumably in response to nutrient fluctuations and too much or too little light (Koufopanou 1994). For the larger colonies, organized flagellar beating also provides stirring of the boundary layer, which facilitates nutrient transfer at rates higher than diffusion alone (Solari et al. 2006a, b). However, there is a constraint between cell division and locomotion imposed by the unique structure of flagella.
These organelles are essentially microtubular assemblages attached to the cell through their basal bodies; they also are connected to the nuclear region where a ‘microtubule organizing center’ (centriole or centrosome in some terminology) plays a key role in cell division by acting as the mitotic spindle. The centers cannot function in both capacities simultaneously. (A similar division and ciliation constraint applies in metazoans; Buss 1987, pp. 35-44.) Flagella can continue to beat without their basal bodies for only about five rounds of cell division (32 cells). Thus, to remain suspended, the larger colonies (implying multiple rounds of cell division) would sink before completing development. An evolutionary solution to this problem was a division of labor whereby such colonies consist of peripheral, sterile, and ultimately mortal somatic tissue with functional flagella to maintain buoyancy and propulsion, supporting non-flagellated, reproductive, immortal germ cells internal to the somatic layer (in Volvox).
Somatic tissue, i.e., differentiation, originates in colonies of 32-128 cells (Koufopanou 1994) and as colony size increases in the Volvocaceae the ratio of somatic to germ cells increases. Detailed hydrodynamic analyses (Solari et al. 2006a, b) following up the work of Koufopanou (1994) show that the relatively increased soma is needed to maintain buoyancy/motility and internalization of germ cells decreases colony drag. A greater allocation of biomass to germ tissue increases colony fecundity but also increases gravitational force and decreases swimming speed. Thus, there are trade-offs among reproduction, motility, and size. Despite these trade-offs, multicellularity along with differentiation arose several times in Volvox, attesting to the advantages of the multicellular and differentiated condition.
Date added: 2025-06-15; views: 217;
