The Microbially-Driven Fenton Reaction: Principles and Environmental Applications
Introduction to Fenton Chemistry for Contaminant Degradation. The hydroxyl radical (•OH), generated through Fenton chemistry, possesses an exceptionally high oxidation potential, enabling it to degrade a wide spectrum of recalcitrant and hazardous environmental contaminants. This capability makes it effective against pollutants commonly found in groundwater, sediments, and industrial wastewater, including landfill leachates, chlorinated aliphatics, and aromatic compounds. Consequently, advanced chemical oxidation processes centered on the chemical Fenton reaction are established treatment strategies for such contaminated sites. The core reaction involves Fe(II) reacting with hydrogen peroxide (H2O2) under acidic conditions to produce Fe(III), a hydroxyl ion, and the potent •OH radical. Despite its efficacy, the classical Fenton process faces significant operational limitations for in-situ remediation.
Limitations of Conventional and Photo-Fenton Systems. A major constraint of traditional chemical Fenton treatments is the continuous, costly external supply of reagents, specifically Fe(II) and H2O2, to sustain •OH radical production. This requirement hinders widespread and economical in-situ application. Alternative systems, such as the photo-Fenton reaction, which uses UV light to regenerate Fe(II) and produce radicals, are also limited. These limitations primarily involve the poor subsurface penetration of UV light and the ongoing need for a continuous input of H2O2. These challenges have driven the exploration of more sustainable, self-sustaining alternatives for environmental remediation.
The Mechanism of the Microbially-Driven Fenton Reaction. The microbially-driven Fenton reaction represents an emerging bioremediation strategy that overcomes the reagent limitation by continuously regenerating the necessary Fenton reagents biologically. This process is typically carried out in bioreactors operated with alternating aerobic and anaerobic phases, amended with Fe(III) and the facultative anaerobic bacteriumShewanella. During the aerobic phase, microbial respiration of oxygen leads to the production of H2O2. Subsequently, in the anaerobic phase, Shewanella reduces Fe(III) to Fe(II). During the transition between these phases, the microbially generated H2O2 and Fe(II) interact to produce •OH radicals, which effectively degrade a diverse range of contaminants, including pentachlorophenol (PCP), trichloroethylene (TCE), and polycyclic aromatic hydrocarbons (PAHs) (Figure 4).
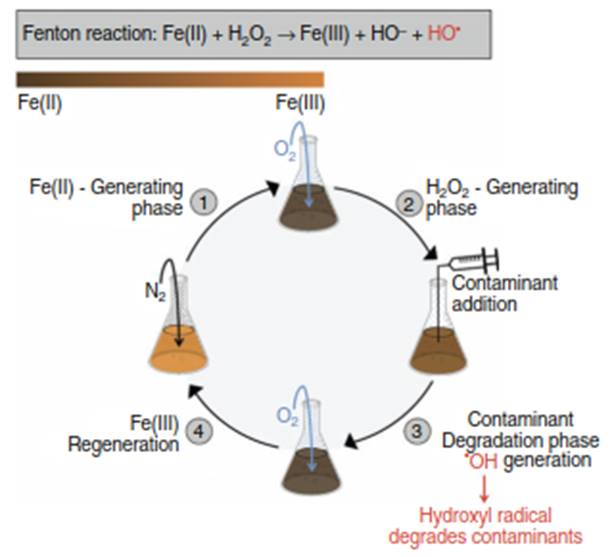
Figure 4. Microbially-driven Fenton reaction drives contaminant degradation in fed-batch reactor systems consisting of four main phases. Phase 1: Batch reactor containing Fe(III) and S. oneidensis cells is incubated under anaerobic conditions to initiate microbial Fe(III) reduction to Fe(II). Phase 2: Under aerobic conditions, S. oneidensis respires aerobically and reduces O2 to H2O2, which reacts with Fe(II) produced in Phase 1 to generate OH radicals. Phase 3: The bioreactor is closed and contaminant (PCP, TCE, PCE, 1,4-dioxane, cellulose, hemicellulose, pyrene, or anthracene) is injected and degraded by the OH radicals generated in Phase 2. Phase4: Fe(III) is regenerated via injection of compressed air and the 4-phase cycle is repeated. The brown-colored bar in the upper left-hand corner indicates the relative reducing (brown-colored, fully Fe(II)) and oxidizing (orange-colored, fully Fe(III)) conditions in the bioreactor
Application in Degrading Chlorinated Organic Pollutants. The effectiveness of this microbial system is demonstrated in the degradation of specific pollutants. For instance, the S. putrefaciens-driven Fenton reaction generates •OH radicals that degrade pentachlorophenol (PCP), a toxic pesticide and wood preservative. Experimental results show approximately 60% PCP degradation, with primary daughter products identified as tetrachlorohydroquinone and tetrachlorocatechol. Similarly, the S. oneidensis-driven Fenton reaction effectively degrades chlorinated solvents like trichloroethylene (TCE) and tetrachloroethylene (PCE), as well as the co-contaminant 1,4-dioxane. The degradation rates for these commingled contaminants directly correlate with their known •OH radical reaction rate constants, confirming the process follows fundamental Fenton reaction principles.
Expansion to Hydrocarbons and Biofuel Feedstock Production. The application of this technology extends beyond chlorinated compounds to include persistent hydrocarbons. Recent studies have successfully employed the S. oneidensis-driven Fenton reaction to degrade recalcitrant oil spill components such as pyrene and anthracene. Furthermore, this system has been repurposed for biofuel production, using lignocellulosic materials from woody plants as a starting substrate. Lignocellulose, composed of cellulose and hemicellulose tightly bound to lignin, is naturally resistant to enzymatic breakdown. However, the Fenton reaction effectively pre-treats this biomass, breaking down the carbohydrate polymers into shorter oligosaccharides and monosaccharides (e.g., glucose and xylose), which can then be efficiently fermented into valuable products like the bioplastic polyhydroxybutyrate (PHB).
Date added: 2025-11-17; views: 161;
