Microbial Bioremediation of Selenium, Tellurium, and Iodine: Mechanisms and Applications
Introduction to Selenium and Tellurium Contamination. Coal and phosphate mining operations are primary anthropogenic sources of selenium and tellurium contamination in wastewater. Furthermore, the combustion of selenium-bearing coals in power plants facilitates the atmospheric release of this element. In both aquatic and terrestrial environments, a significant biogeochemical process is the microbially-driven reductive methylation of selenium and tellurium. The most prevalent volatile methylated forms produced are dimethyl selenide (DMSe) and dimethyl telluride (DMTe). These organic species are critical for understanding the natural cycling and detoxification of these elements.
Microbial Methylation and Detoxification Mechanisms. A key enzyme in this process is the bacterial thiopurine methyltransferase (bTPMT), which catalyzes the methylation of selenite and (methyl)selenocysteine into dimethylselenide (DMSe) and dimethyldiselenide (DMDSe). This enzyme performs analogous transformations on tellurite oxyanions. The biological significance of this methylation pathway is profound; methylated selenium and tellurium derivatives are not only volatile but also exhibit significantly lower toxicity compared to their inorganic forms. Concurrently, the direct enzymatic reduction of selenite or tellurite to amorphous elemental Se(0) or Te(0) represents a parallel strategy for immobilization and detoxification. Genomic evidence suggests that subsequent multiple methylation steps of the reduced Se or Te may be catalyzed by currently unidentified thiopurine methyltransferases encoded in the S. oneidensis genome (Figure 3).
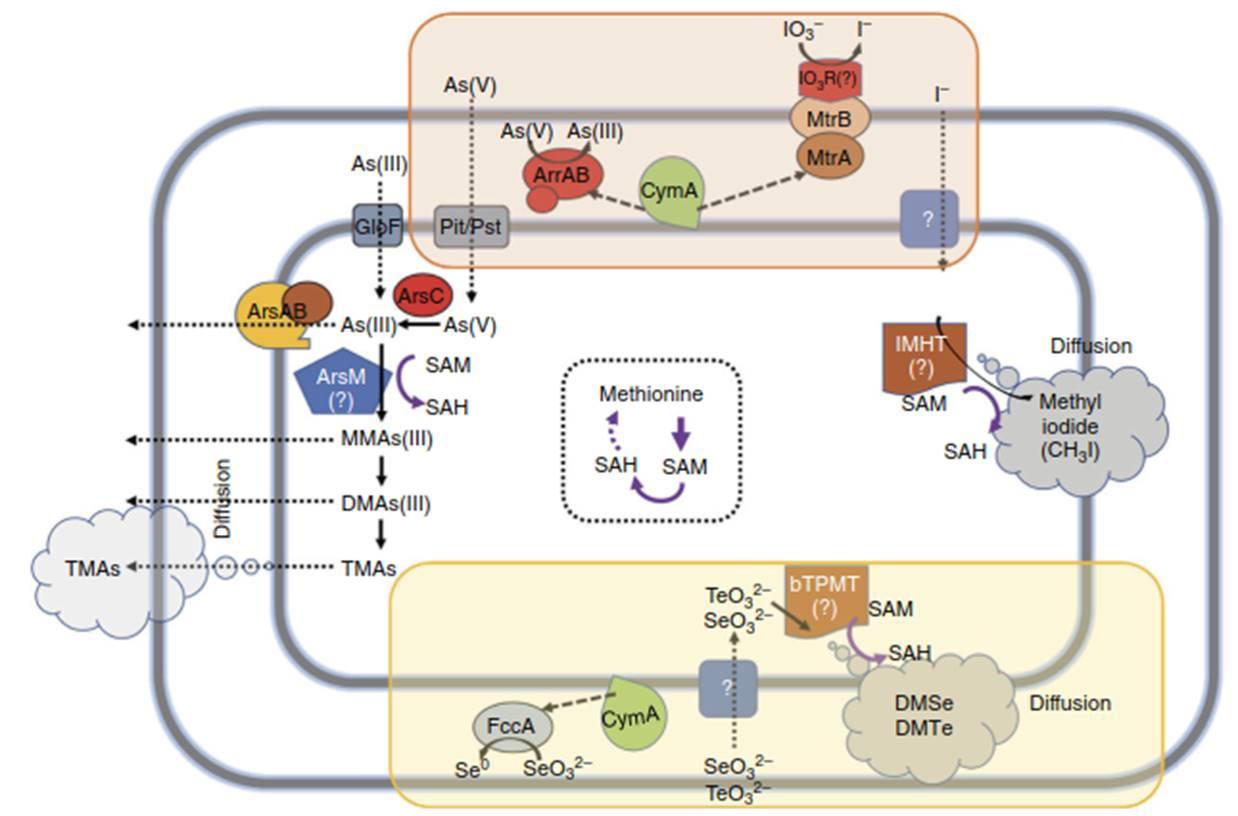
Figure 3. Reductive methylation pathways predicted from genomic analysis of the S. oneidensis genome. Orange: Working model of reductive methylation of arsenate: periplasmic ArrAB reduces an extracellular As(V) to As(III) which is imported by Pit/Pst into the cytoplasm. Cytoplasmic As(V) could be reduced to As(III) by ArsC and exported extracellularly by ArsAB or transformed to methylated arsenic compounds by sequential methylation of ArsM. Yellow: Working model of reductive methylation of selenite or tellurite: periplasmic FccA reduces selenite (SeO32-) to Se0, via a yet unknown mechanism. Selenite(SeO32-) and tellurite(TeO32-) are imported by unknown permeases and transformed to gaseous methylated forms, DMSe or DmTe by bTPMT. Green: working model of reductive methylation of iodate: IO3- is reduced to I- by an extracellular IO3--reductase complex composed of MtrA, MtrB and an unidentified terminal IO3- reductase (IO3R).
Subsequently, the reduced I- is converted to methyliodide (CH31) by an unidentified iodide-specific methylhalide transferase (IMHT) after cytoplasmic import of I-. Abbreviations: ArrAB, arsenite respiratory reductase; GlpF, glycerol transporter; Pit/Pst, phosphate transporter; CymA, tetraheme c-type cytochrome; ArsAB, arsenite detoxification efflux pump; ArsC, arsenate detoxification reductase; FccA, fumarate reductase; MtrA, MtrB, IO3R, Iodate reductase; IMHT, iodide-specific methylhalide transferase; bTPMT, bacterial thiopurine methyltransferase; DMSe, dimethylselenide; DMTe, dimethyltelluride; SAM, S-Adenosyl methionine; SAH, S-Adenosyl homocysteine
Reductive Precipitation as a Remediation Technology. Similar to established bioremediation strategies for uranium and technetium, the microbial reductive precipitation of selenium and tellurium oxyanions offers a promising technological application. While the reduction of selenate (SeO₄²⁻) is less common and can support anaerobic growth for some organisms, the reduction of selenite (SeO₃²⁻) is a more widespread environmental process. This reduction of SeO₄²⁻ to elemental Se(0) constitutes a major sink for selenium oxyanions in anoxic sediments. Notably, bacteria within the Shewanella genus demonstrate a remarkable capability to reduce both selenate and tellurate (TeO₄²⁻), as well as selenite and tellurite (TeO₃²⁻), precipitating them as elemental Se(0) and Te(0).
Molecular Pathways of Selenite and Tellurite Reduction. Recent research has identified fumarate reductase (FccA) in S. oneidensis as the terminal selenite reductase located in the periplasmic space. This enzymatic reduction also involves CymA, a crucial c-type cytochrome central to the anaerobic respiratory chain of Shewanella. A key distinction in the reduction pathways is observed in the localization of the resulting precipitates; selenium forms extracellular precipitates, whereas tellurium typically accumulates intracellularly. This differential localization strongly suggests that SeO₃²⁻ and TeO₃²⁻ are processed by separate and distinct electron transport pathways within the cell.
Iodine Biogeochemistry and Radioactive Remediation. The microbial reduction of iodate (IO₃⁻) is a fundamental component of the global biogeochemical cycling of iodine, occurring in marine, terrestrial, and contaminated environments. This process is particularly relevant for the bioremediation of the long-lived radioactive fission product Iodine-129 (¹²⁹I), which has a half-life of 15.7 million years. The iodine cycle is a complex network integrating both abiotic (chemical) and biotic (enzymatic) reactions. In a typical marine sequence, IO₃⁻ is first reduced to iodide (I⁻) by specialized iodate-reducing microorganisms.
Volatilization and Completion of the Iodine Cycle. The produced iodide (I⁻) is subsequently volatilized from surface waters by various algae and bacteria through methylation. This biological methylation yields volatile organic iodine compounds such as methyl iodide (CH₃I), iodomethane (CH₂I₂), iodoethane (C₂H₅I), and iodopropane (C₃H₇I). The biogeochemical cycle is completed when I⁻ is oxidized back to IO₃⁻, a process initiated by iodide-oxidizing microorganisms that convert I⁻ to iodine (I₂), which then hydrolyzes and disproportionates to re-form iodate.
Molecular Mechanisms of Iodate Reduction and Methylation. The precise molecular mechanism of microbial IO₃⁻ reductive methylation is still being elucidated. It is known that SAM-dependent methyl halide transferases from diverse organisms catalyze the methylation of I⁻ to methyl iodide (CH₃I). The reductive methylation of IO₃⁻ has been documented in Shewanella putrefaciens strain MR-4, while methylation of I⁻ is performed by S. putrefaciens IAM 12079. Among Shewanella species, S. oneidensis MR-1, S. putrefaciens 200, and Shewanella algae BrY exhibit the highest rates and extents of IO₃⁻ reduction.
Key Genes and a Proposed Model for Iodine Processing. Genetic studies in S. oneidensis MR-1 indicate that IO₃⁻ reduction involves key metal-reduction components including MtrA and MtrB (though not MtrC), as well as the catabolite repressor protein (Crp) and cytochrome maturation proteins. The genome also harbors fourteen putative SAM-dependent methyltransferases, which are candidates for catalyzing the final methylation step. A working model, integrating phenotypic and genomic data, proposes that IO₃⁻ is first reduced to I⁻ by an extracellular terminal reductase complex containing MtrA and MtrB. The resulting iodide is then converted to methyliodide (CH₃I) by a specific, yet-to-be-identified iodide-specific methylhalide transferase (IMHT) (Figure 3).
Date added: 2025-11-17; views: 176;
