Shewanella Electron Transfer: Mechanisms for Bioremediation of Metals and Radionuclides
Heavy metals, metalloids, and radionuclides pose a significant environmental threat due to their toxicity and potential to accumulate in living organisms [13]. Remarkably, bacteria from the genus Shewanella can transform a wide array of these toxic elements, including uranium and technetium [14]. This bioremediation capability often relies on a unique process known as Extracellular Electron Transfer (EET). EET is a critical component of anaerobic respiratory pathways that allow bacteria to reduce electron acceptors which are physically unable to enter the cell and interact with the standard, inner membrane-localized electron transport chain used in aerobic respiration [15].
Extracellular Electron Transfer (EET) is Shewanella's solution to a fundamental problem: insoluble metal oxides like Mn(IV) and Fe(III) cannot cross the cell membrane. To overcome this, the bacterium transports electrons from internal metabolic processes directly to these external solid acceptors [9, 16, 17]. This process is facilitated by a sophisticated biochemical machinery. It begins with dehydrogenases that oxidize electron donors and transfer electrons to menaquinone within the inner membrane (Figure 2). From there, electrons flow through a series of multi-heme c-type cytochromes, starting with CymA in the inner membrane.
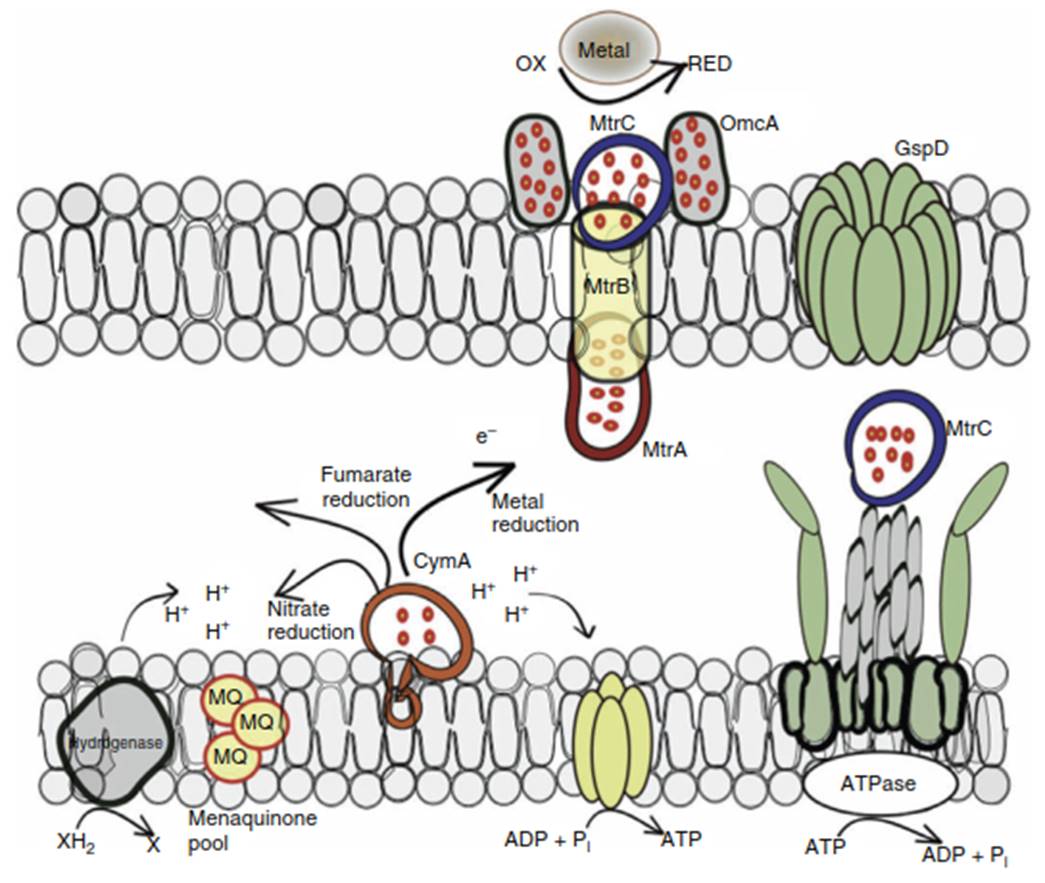
Figure 2. Working model of the S. oneidensis respiratory pathway for electron transfer to extracellular electron acceptors
The electron transfer chain continues into the periplasm with the decaheme cytochrome MtrA. The outer membrane protein MtrB acts as a conduit, facilitating interaction between MtrA and the outer membrane decaheme cytochrome MtrC. Together, they form the MtrCAB complex, which functions as a molecular wire, shuttling electrons across the outer membrane to solid-phase acceptors like Fe(III) oxides [18]. This complex is strategically assembled at the cell surface, with MtrC being secreted and anchored to the outer membrane to directly interact with the extracellular environment.
Shewanella employs several innovative strategies to deliver electrons to external Fe(III) oxides. These include: (i) direct reduction via cell-surface localized c-type cytochromes like MtrC [6, 19, 20]; (ii) electron transport along extracellular nanowires that are decorated with cytochromes; (iii) the use of endogenous or exogenous soluble electron shuttles [21-24]; and (iv) the nonreductive dissolution of Fe(III) oxides to form more easily reducible soluble organic complexes [25-29]. This metabolic flexibility allows Shewanella to thrive in diverse and challenging subsurface environments.
The bioremediation potential of Shewanella is particularly valuable for addressing subsurface contamination by radionuclides like uranium (U) and technetium (Tc), byproducts of nuclear fuel processing. The mobility of these contaminants is highly dependent on their oxidation state. Under oxidizing conditions, U(VI) and Tc(VII) form soluble, mobile complexes. Under reducing conditions, they are transformed into U(IV) and Tc(IV) oxides, which are highly insoluble and far less mobile [30]. This principle allows dissimilatory metal-reducing bacteria to be used in strategies designed to immobilize contaminant plumes in groundwater [31, 32].
Research has confirmed that the outer membrane cytochromes MtrC and OmcA, key to Fe(III) and Mn(IV) reduction, are also directly involved in reducing U(VI) and Tc(VII). Mutant strains lacking the mtrC and omcA genes show severely impaired U(VI) reduction and less association of the insoluble product UO2 with the cell surface [33]. The subsequent reactivity and potential re-oxidation of these biogenic UO2 nanoparticles are strongly influenced by their association with natural biopolymers in the environment [33]. Furthermore, the presence of strong organic chelators like citrate or EDTA can retard precipitation by forming soluble U(IV)-ligand complexes, with reduction rates inversely related to the stability of the U(VI)-ligand complex [34].
Date added: 2025-11-17; views: 160;
