Cellular Ion Transport Mechanisms
Mineral nutrients in the soil solution are practically always present as ions and carry an electric charge. Thus, they cannot cross biological membranes at sufficiently high rates without the involvement of proteins that form specialised pores allowing passage through the membranes. Ions can move into the cytosol and out of the cytosol. Movement from the exterior into the cytosol is called uptake; transport into the extracellular space is called efflux. All other transport processes occur across organellar membranes.
Most relevant for mineral nutrition are uptake, efflux and transport into and out of the vacuole (Fig. 7.8). A large number of transport proteins mediating these processes are encoded in plant genomes, as we know from the model species A. thaliana and rice. For example, around 50 genes encode K+ channels and K+ transporters in A. thaliana alone.
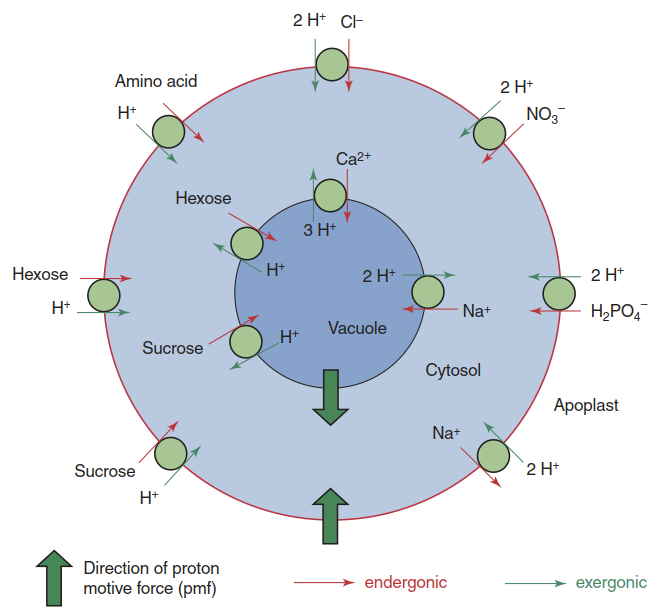
Fig. 7.8Secondary active transport across the plasma membrane and the tonoplast. The proton motive force (pmf) generated by proton pumps and pyrophosphatases provides the energy for transport against an electrochemical potential gradient. A few examples of myriad transport processes in a plant cell are shown. (Modified from Weiler and Nover (2008))
Depending on the driving force, three categories of transporters are distinguished (Fig. 7.9). Facilitated diffusion refers to transport that is energetically favourable because it occurs along an electrochemical potential gradient. Typical proteins enabling facilitated diffusion are channels such as K+ channels (Figs. 7.11 and 7.12).
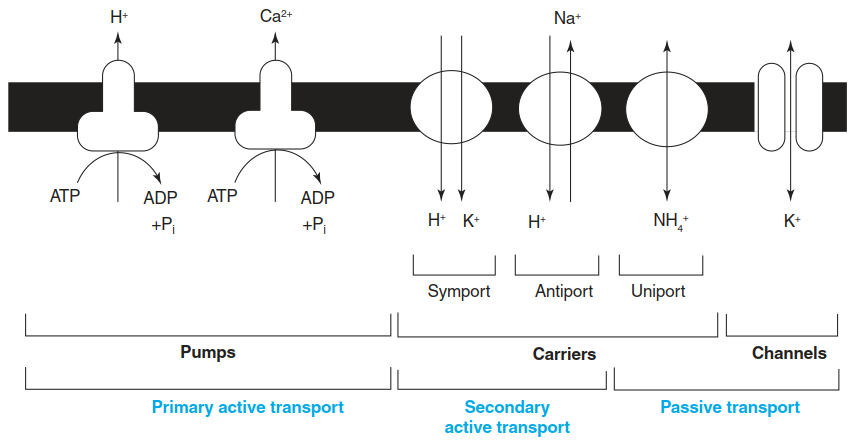
Fig. 7.9. Different categories of transport. (Modified from Marschner (2012))
Active transport moves an ion (or a metabolite) against an electrochemical potential gradient—for example, from the soil solution with a low concentration to the cytosol of a root cell with a high concentration (enrichment)—or an anion against the negative potential of the plasma membrane (by definition, the membrane potential of a cell is negative when there is a surplus of negative charges on the cytosolic side). Primary active transport is directly energised by the hydrolysis of adenosine triphosphate (ATP). Secondary active transport uses the energy supplied by a gradient in electrochemical potential through coupling of the energetically favoured movement of one molecule to the unfavourable movement of another (Fig. 7.9).
The dominant primary active transport in plant cells is the establishment of a proton gradient across the plasma membrane and the tonoplast by the activity of H+-ATPases (and additionally of pyrophosphatases in the case of the tonoplast). This proton gradient, the proton motive force, provides the driving force for myriad transport processes in plants (Fig. 7.10). It can be used by carrier proteins (Fig. 7.9).
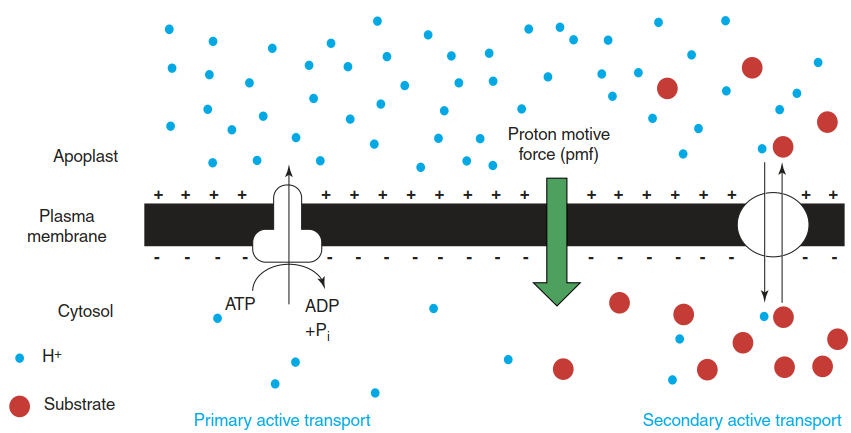
Fig. 7.10. Primary active transport fuels secondary active transport. The pumping of protons from the cytosol to the apoplast is a primary active transport, directly energised by ATP and mediated by P-type H+-ATPases. It contributes to the particularly negative membrane potential of plant cells. The resulting proton motive force can drive secondary active transport through the coupling of energetically favoured proton influx with, for example, efflux of a positively charged substrate against an electrochemical potential difference
Many of them co-transport protons with ions or metabolites. This co-transport can be a symport (i.e. both molecules move in the same direction) or an antiport (i.e. the molecules move in opposite directions). While most carriers involved in nutrient acquisition couple transport to the movement of protons and are therefore secondary active, there are many others that couple the transport of two metabolites or of a metabolite and an ion and are passive. Examples are the phosphate translocator in the inner plastid membrane, which exchanges phosphate with 3-phosphoglycerate, or the malate/oxaloacetate shuttle. A third type of carrier—and passive too—is the uniporter that mediates transport of one molecule along a potential gradient.
The activities of cation and anion channels depend on the cell’s external and internal ion concentrations, which establish a specific membrane potential (Fig. 7.10). The so-called rectifying channels allow charges (ions) to pass more easily either into the cells (inward rectifier) or in the outward direction (outward rectifier), thus also affecting the membrane potential (Fig. 7.11). The same holds for channels in the tonoplast membrane. At a membrane potential negative to the equilibrium potential of, for example, K+, inward potassium rectifiers support the flow of the potassium cation into the cell, rendering the membrane potential more positive until the equilibrium potential (also termed the Nernst potential) of K+ is reached. Anion-rectifying channels operate in a similar way with anions.
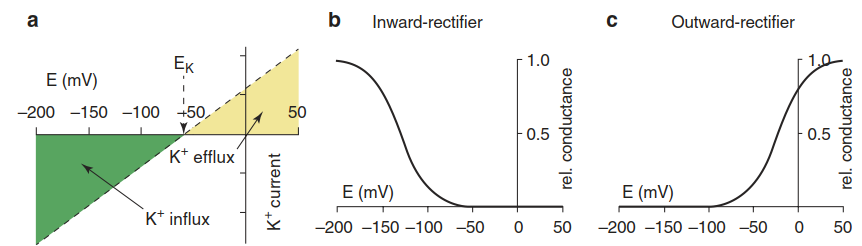
Fig. 7.11. Dependency of K+ channels on the membrane potential (voltage). a The dashed line shows the degree to which a K+ channel population is open for inward or outward K+ fluxes. At the equilibrium voltage (EK) no net flux of potassium takes place. The EK depends on the potassium concentrations on both sides of the membrane. In principle, and given a respective membrane potential, an inward rectifier can also export K+ ions, and vice versa. The activity of the voltage-gated channels changes gradually with a change in the membrane potential. Inward-rectifying K+ channels b open upon hyperpolarisation of the membrane potential (i.e. a shift to more negative values), and outward-rectifying K+ channels c open upon depolarisation of the membrane potential (i.e. a shift to less negative values). (Dreyer and Blatt 2009)
Channels whose activities change the membrane potential can, on the other hand, be controlled by that potential. They are termed voltage-gated channels, in contrast to channels that are controlled by specific ligands (ligand-gated channels). Gating of these channels can control K+ mineral nutrition (Sect. 7.3.2.2), signalling, and abiotic as well as biotic stress responses. The regulation of inward- and outward-rectifying K+ channels plays a key role in controlling the turgor of guard cells and the apertures of stomata (Sect 6.3.3).
Depending on the nutrient ion in question, uptake into the root symplast—that is, the cytosol—has to be either energised or not. The plasma membrane potential of plant cells is negative (around -150 mV), owing largely to the proton pumping activity of H+-ATPases. Thus, cations such as K+ or Fe2+ can in principle move passively into the cytosol along an electric potential gradient through channels or uniporters. In contrast, anions such as phosphate, nitrate and sulphate enter a root cell against a potential gradient. This is enabled by H+-coupled symport (Figs. 7.8 and 7.10). Conversely, efflux out of the symplast into the apoplast for xylem loading requires energisation for the cations and is energetically favourable for the anions.
One of the hallmarks of plant mineral uptake is the existence of multiphasic uptake systems with varying affinities, as classically shown for K+ (Epstein et al. 1963). Depending on the concentration in the soil solution, low-affinity or high-affinity transport systems with affinities in the millimolar or micromolar range, respectively, are in operation (Fig. 7.12).
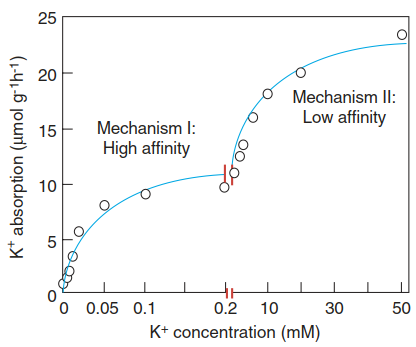
Fig. 7.12. Multiphasic nutrient uptake systems. Usually two types of uptake systems for nutrients such as K+ can be expressed in plants, depending on availability and the physiological state; a high-affinity system is induced when nutrient availability is limited, and a low-affinity system operates when the nutrient supply is good
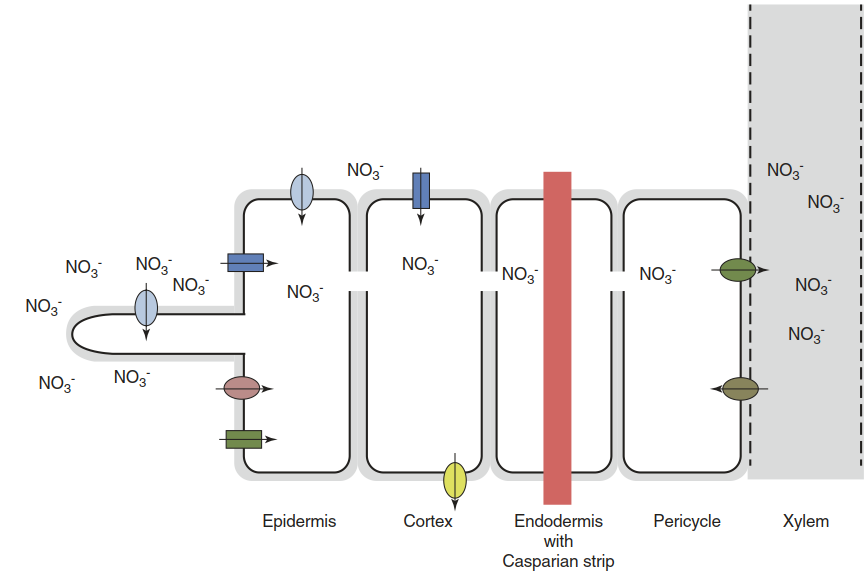
Fig. 7.13. Nutrient acquisition by the plant root is dependent on families of transporters differing in affinity, expression level and localisation. The example of nitrate is shown. Both uptake and efflux activities are involved in supplying nitrate in the right concentrations to roots and—via the xylem—shoots. Ovals represent members of the low-affinity NRT1 transporter family; rectangles represent members of the high-affinity NRT2 transporter family. The storage of nitrate in root cell vacuoles is not shown
As for enzymes, the affinity for the substrate is expressed as a KM (Michaelis-Menten) value—that is, the substrate concentration at which the transport rate is half maximal. Typically, a plant possesses several isoforms of both low-affinity and high- affinity transporters, which vary in localisation and timing of expression (Fig. 7.13). This applies to both monocot and dicot plants.
Date added: 2025-01-27; views: 518;
