Elements in the Soil
The soil represents an immensely complex physical, chemical and biological substrate. Nutrient availability strongly varies in space and time. Soil types differ tremendously in mineral content. Large and element-specific fluctuations occur within a soil—for instance, depending on changes in pH (Fig. 7.3), water status or microbial activity. Gradients develop horizontally and vertically. Elements can be found in patches because of uneven distribution of factors influencing availability—for example, litter fall and decomposition. Mobility within the soil is strongly element-specific.
Soil consists of solid, liquid and gaseous phases. The mineral nutrient supply is influenced by all three phases. The solid phase contains most of the nutrients. Inorganic soil matter is a reservoir for nutrients such as K and Fe, and organic soil matter is the main N reservoir. Minerals originate mostly from the weathering of bed- rock—the sediments that give rise to soil. The exception is nitrogen, which predominantly stems from nitrogen fixation. Weathering rates are strongly influenced by age and environmental factors. For instance, many tropical soils are highly weathered and the soils are therefore depleted in plant-available phosphorus.
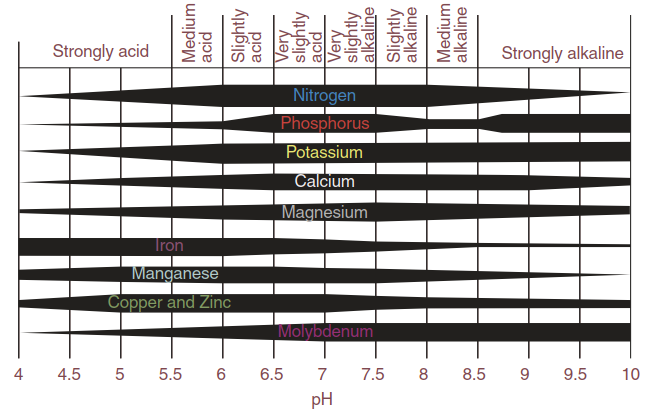
Fig. 7.3. Influence of soil pH on the availability of different mineral nutrients. (Lambers et al. 2008)
The liquid phase contains ions available for uptake by roots. Only a very small fraction of total soil minerals are in soil solution. The bulk is bound to soil particles (e.g. clay, humic acids) that carry mostly negative charges, thus providing binding sites for cations. Minerals are in a dynamic equilibrium between the two phases. Ion exchange processes result in slow release into the soil solution. The binding capacity of the particles is an important soil fertility factor, and the average particle diameter is therefore a parameter used for soil classification. The high binding capacity of smaller particles reduces leaching and thus increases reserves.
While roots tap nutrient resources by growth, nutrient ions ultimately have to reach the root surface via mass flow and diffusion. Elements differ strongly in their mobility within the soil solution. Phosphate is about three orders of magnitude less mobile than nitrate or sulphate (Lambers et al. 2015) because of stronger interaction with soil particles. This has major consequences for the biology of nutrient acquisition. About 90% of all plant species live in a mycorrhizal symbiosis with fungi. The fungal hyphae greatly enhance the capacity to unlock immobile phosphate in the soil.
Soil particles form pores that differ in size between macro- and micropores. They are partly filled with water and partly with air, depending on the type of the soil and the amount of precipitation (Fig. 5.1). Air-filled pores are important for the gas exchange of the respiring roots (autotrophic respiration) and of other soil organisms (heterotrophic respiration). Gas exchange of roots and soils influences nutrient availability. For example, CO2 released from roots as the product of respiration dissolves in H2O and forms hydrogen carbonate (HCO3-) and H+. These ions can desorb nutrient ions from soil particles through ion exchange and thereby enhance bioavailability.
Strong variation exists between species and also within species (i.e. between ecotypes, cultivars, varieties) in their ability to acquire nutrients from soil. Thus, soil mineral availability has a strong influence on the distribution and composition of natural vegetation (Marschner 2012) (compare global soil map Fig. 11.2). This is illustrated by widely used classifications such as calcicoles versus calcifuges—that is, plants thriving on alkaline limerich soil versus plants with a preference for acidic soil. Some plant species have evolved specific adaptations to particularly nutrient-impoverished soils— for example, highly weathered ancient soils in Australia and South Africa, or soils in cold climates with very slow mineralisation of organic matter.
Date added: 2025-01-27; views: 381;
