Nutrient Acquisition and Responses to Nutrient Scarcity
As mentioned, nutrient scarcity is a general theme of a plant’s life. The hidden half of plant biology—that is, the biology of the root—can, by and large, be explained by the need to acquire— besides water—the mineral nutrients essential for growth. A whole array of physiological, biochemical and developmental processes operate to modulate theavailability of nutrients, to allow uptake from the soil solution, to store and to distribute to other organs or to engage in symbioses that greatly facilitate nutrient acquisition.
There are common themes applicable to most or all nutrients (e.g. the existence of specialised transporters in the plasma membrane), as well as mechanisms specific to one or a few nutrients (e.g. the modulation of the rhizosphere to mobilise scarcely available Fe(III)). Those molecularly best understood are the acquisition of the macroelements P, N and K, and the microelement Fe. They will therefore be the main focus of Sect. 7.4 and will be discussed to illustrate the principles of plant root responses to the stress of nutrient scarcity.
Four principal strategies that plant roots use to ensure adequate nutrient acquisition can be distinguished (Fig. 7.4):
- They influence the availability of nutrients in the rhizosphere
- They tightly regulate ion transport capacities
- They modulate their architecture—that is, the three-dimensional morphological structure
- They establish symbioses with fungi (mycorrhizae) and bacteria (biological N2 fixation)
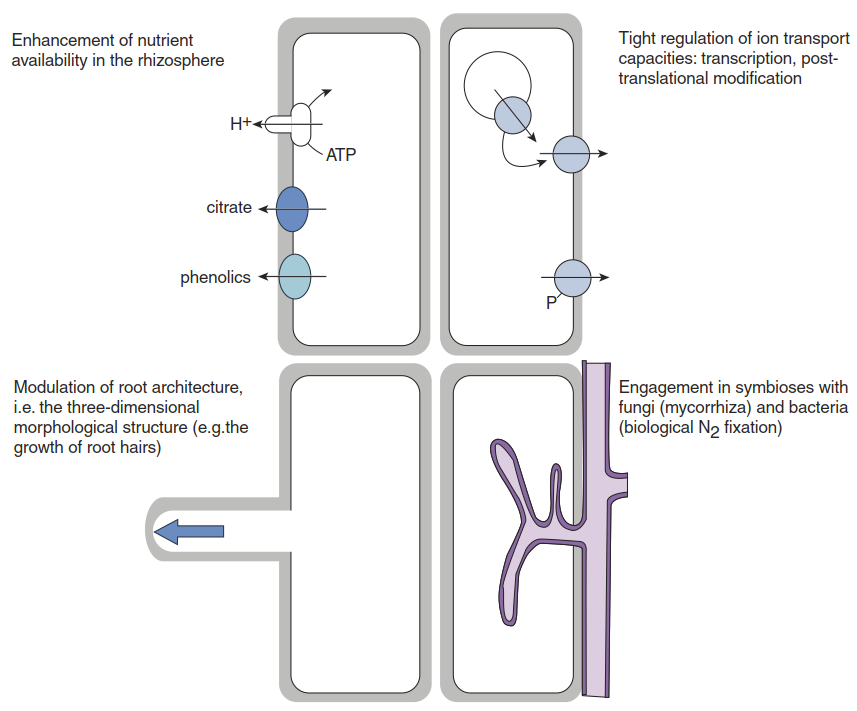
Fig. 7.4. Strategies for nutrient acquisition
The molecular aspects of these strategies are detailed in Sects. 7.3.1-7.4.3.
Modulation of Nutrient Availability. Plant roots actively influence the rhizosphere— that is, the immediate vicinity of the roots—in order to change the availability of nutrients. Because of the important role of soil pH, acidification by proton pumping is one prevalent mechanism that enhances availability of Fe, Zn, B and Mn. Organic acids such as malate and citrate are among the major components of root exudates. Deposition of carbohydrates in the rhizosphere accounts for a substantial fraction of the 20-60% of photosynthetically fixed carbon that is transferred below-ground by plants (Kuzyakov and Domanski 2000).
Release of organic acids can occur passively through damaged root cells and via controlled secretion through anion channels in the plasma membrane. Stimulation of the latter has been observed especially in response to phosphate deficiency. Citrate and other carboxylates can mobilise sparingly soluble phosphate adsorbed to Fe or Al oxides. Their exudation is particularly pronounced in cluster roots of certain plants adapted to phosphate-poor soils (Fig. 7.19). Phosphate can in addition be mobilised by the secretion of enzymes (e.g. phosphatases) that can hydrolyse organic P esters (Fig. 7.5).
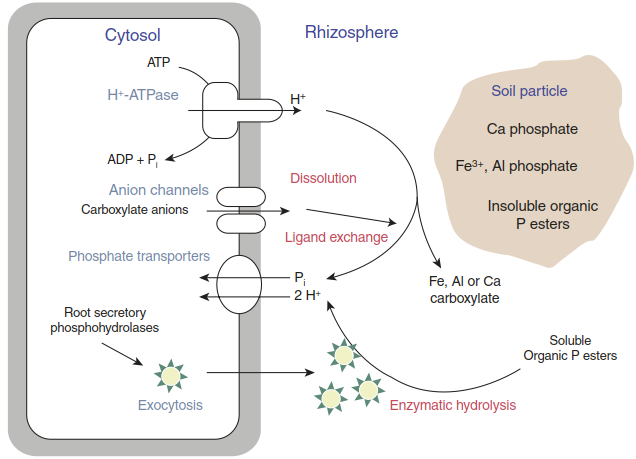
Fig. 7.5. Roles of root exudation in phosphate acquisition. (Modified from Neumann and Martinoia (2002))
Because of the complexity of the processes and the difficulties in experimentally accessing the rhizosphere, detailed molecular understanding of root exudates and their contribution to nutrient availability is still limited. In contrast, a well-understood and very important example not only of nutrient mobilisation is Fe acquisition (Kobayashi and Nishizawa 2012), which will therefore be a recurring theme throughout this chapter. Iron is a particularly problematic element with respect to bioavailability.
The usage of Fe as a redox-active element in biological systems evolved at a time when conditions in the Earth’s atmosphere were reducing, making Fe readily available because Fe sulphides are highly soluble (Frausto da Silva and Williams 2001). With the advent of oxygenic photosynthesis, the bioavailability of Fe gradually and dramatically dropped by about eight orders of magnitude. In an oxidising atmosphere, Fe is mostly present as insoluble Fe oxides. Massive Fe precipitation resulted in the formation of red bands in sedimentary rock about 2.5 billion years ago. Thus, Fe is one of the most abundant elements in the Earth’s crust, yet it is scarcely available in many habitats because it is mostly present in the oxidised form Fe(III), which is barely soluble, especially at pH values above neutral (Fig. 7.3).
Terrestrial plants evolved two distinct strategies to mobilise and to take up Fe (Romheld and Marschner 1986). Strategy I, expressed by dicots and non-graminaceous monocots, consists of subsequent acidification, reduction and uptake steps. Protons are secreted into the rhizosphere by proton pumps (P-type H+-ATPases) to enhance solubility of Fe(III). Plasma membrane-localised ferric reductases reduce Fe(III) chelate complexes to Fe(II), which is then taken up into root epidermal and cortex cells. This strategy is supplemented by the secretion of phenolic compounds such as coumarins, which may act as chelators and/or reductants of Fe(III) (Clemens and Weber 2016). Strategy II is characteristic for grasses. Fe(III)- chelating phytosiderophores such as mugineic acids are secreted by root cells. Phytosiderophores form complexes with Fe(III), which are substrates for specialised transporters that mediate the uptake of these complexes. Many of the proteins mediating these processes have been molecularly identified in Arabidopsis thaliana and maize. Loss-of-function mutants have demonstrated the essentiality of the different steps for growth under Fe-limited conditions. Maize mutants lacking the phytosiderophore uptake transporter Yellow stripe 1 (ys1) show characteristic Fe deficiency-caused chlorosis phenotypes of the leaves (Curie et al. 2001) (Fig. 7.6).

Fig. 7.6. Chlorosis of the Yellow stripe1 maize mutant. The ys1 mutant (left) suffers from Fe deficiency because of a defect in an Fe(III)–phytosiderophore complex transporter. (Photo courtesy of University of Massachusetts Amherst)
The synthesis and secretion of Fe-chelating molecules is strongly activated under Fe limitation. In rice, barley and maize, as representative grass species, several genes encoding enzymes of the mugineic acid synthesis pathway are up-regulated. Examples include nicotianamine synthase (NAS), nicotianamine aminotransferase (NAAT) and deoxymugineic acid synthase (DMAS) (Fig. 7.7).
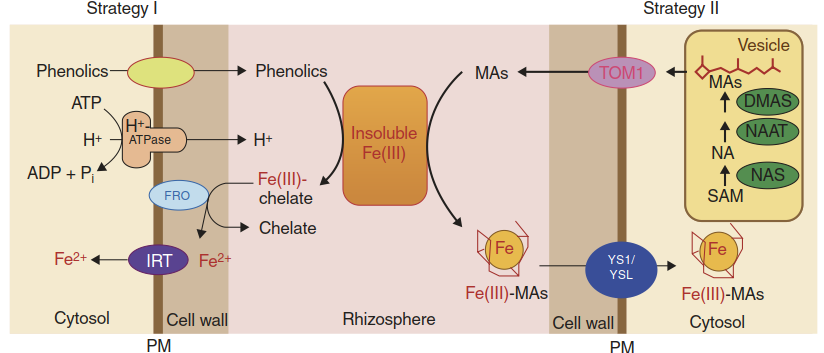
Fig. 7.7. The two strategies for Fe acquisition. Higher plants, with the exception of grasses, use strategy I: a combination of acidification by protein pumping, the reduction of Fe(III) by ferric reductases (FRO) and uptake by Fe(II) transporters (IRTs). Especially when the soil pH is alkaline and acidification is difficult, the secretion of phenolics helps improve Fe availability. Grasses employ strategy II: the secretion of Fe(III)-chelating molecules—the phytosiderophores of the mugineic acid (MA) family—by transporters such as TOM. Fe(III)–MA complexes are then taken up by transporters of the YS1/YSL family. SAM S-Adenosylmethionine, further explanations in the text. (After Kobayashi and Nishizawa (2012))
The same applies to the transporters for mugineic acid or Fe(III)-mugineic acid complexes. Variations within and between grass species in the ability to thrive on alkaline soil have been explained by differences in phytosiderophore secretion rates. Barley shows much stronger mugineic acid release than rice. When rice was engineered to produce more phytosiderophores through transfer of a more strongly expressed nicotinamine aminotransferase gene from barley, growth and yield on alkaline soil was significantly improved (Takahashi et al. 2001)—an example for the engineering of stress tolerance.
It is important to note that P and Fe are merely the two best-understood examples of active modulation of nutrient availability. The aforementioned rhizodeposition of organic compounds strongly influences the density and the activities of microbial communities around the root. These activities in turn have pronounced effects on nutrient chemistry. The questions as to whether and how plant roots actively recruit certain microbial populations (the rhizosphere microbiome) can only now begin to be addressed, owing largely to the revolution in DNA sequencing technologies (Bulgarelli et al. 2013).
Date added: 2025-01-27; views: 335;
