The Nitrogen Cycle: Microbial Processes, Valence States, and Global Impact
Nitrogen (N) is a versatile element commonly found with nine different valence states, as detailed in Table 1. Its most reduced form possesses a chemical valence of -3, found in compounds like ammonia (NH3) and amino groups, while its most oxidized form has a valence of +5, as in nitrate (NO3-). These various N species are interconnected through biological redox reactions, which convert more oxidized forms to more reduced ones in reducing environments, and vice versa. This multiplicity of valence states has facilitated the evolution of a diverse consortium of microbes, each specialized in one or more specific N transformations. The ability of nitrogen to exist in both gaseous and dissolved forms contributes to the notable complexity of its global biogeochemical cycle, illustrated in Figure 1.
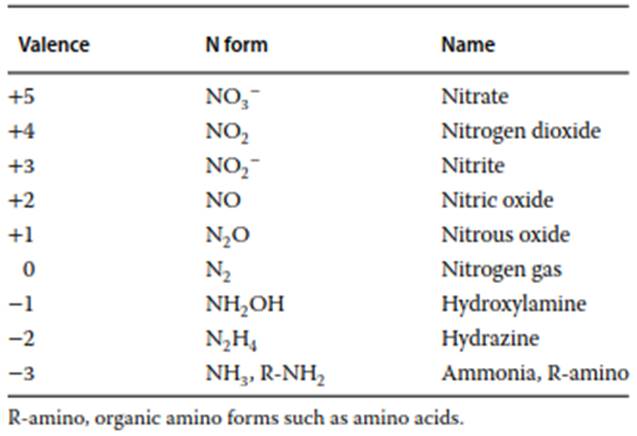
Table 1. Common forms of N and their valence states
The nitrogen cycle is primarily driven by key microbial processes, including N-fixation, nitrification, denitrification, assimilation, and ammonification. Nitrogen fixation is the microbial reduction of inert dinitrogen gas (N2) to ammonia (NH3), a process often occurring in symbiotic association with plants (Figure 1), though free-living N-fixers also exist. Broadly, the term "N fixation" can encompass any process converting N2 to bioavailable forms, including anthropogenic production of nitrogen oxides via combustion and the industrial Haber-Bosch process for fertilizer manufacturing. By this expanded definition, human activity constitutes a major source of fixed nitrogen. While the availability of fixed N often limits primary production in natural ecosystems, the ammonia generated can be oxidized to nitrate or directly utilized by biota.
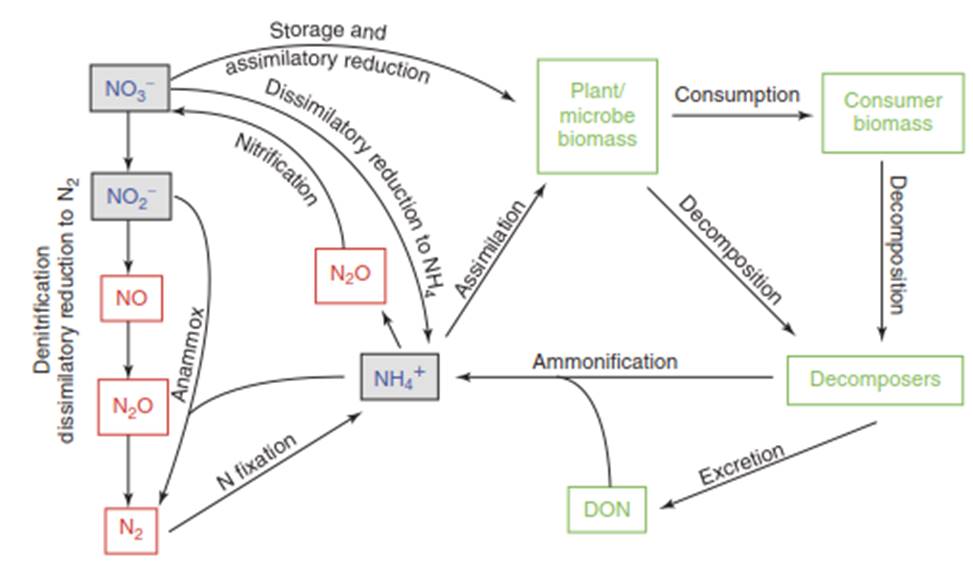
Figure 1. The N cycle in soil, sediments, and water. Organic N is shown as green, ions are blue, and gases are red. Dissimilatory reduction to N2 includes annamox, AOMD, and anaerobic oxidation of H2S (see text). The large range of valences for N (Table 1) provides energetic pathways for microbes to exploit along redox gradients
The process of nitrification involves the oxidation of ammonia (NH3) to nitrate (NO3-), typically an aerobic, energy-yielding process for microorganisms that also produces nitrous oxide (N2O) as a minor by-product. The nitrate produced can be stored in plant vacuoles for later use or assimilated by plants and microbes. However, assimilation into organic forms requires the energy-intensive enzymatic reduction of nitrate back to ammonia. Alternatively, nitrate can be reduced to gaseous nitrogen forms—nitric oxide (NO), nitrous oxide (N2O), or dinitrogen gas (N2)—through denitrification. This process is a form of anaerobic respiration where microorganisms use nitrate as a terminal electron acceptor in the absence of oxygen.
A key alternative pathway is the dissimilatory nitrate reduction to ammonia (DNRA), an anaerobic process that converts nitrate to ammonia, thereby removing nitrate from the environment while retaining reactive nitrogen within the soil system. Ammonia is also regenerated through the decay of organic matter by bacteria and fungi, a process known as ammonification (often called N mineralization). The dissolved gas ammonia (NH3) equilibrates with water to form ammonium hydroxide (NH4OH), which dissociates into the ammonium ion (NH4+) and a hydroxide ion. The speciation is pH-dependent, with NH4+ predominant at acidic pH (<5) and volatile NH3 prevailing at alkaline pH (>9), where it can be lost to the atmosphere.
Beyond classic denitrification, at least three other biological processes reduce oxidized nitrogen to dinitrogen gas (N2). The first is anammox (anaerobic ammonium oxidation), a transformation where nitrite (NO2-) and ammonium (NH4+) combine directly to form N2. The second is the anaerobic oxidation of methane by denitrification (AOMD), which couples the C and N cycles by linking methane oxidation to the reduction of nitrite and nitrate (Figure 1). A third process involves the anaerobic oxidation of sulfide coupled to denitrification, creating a link between the S and N cycles. For simplicity, these three processes can be collectively termed "dissimilatory nitrate reduction to N2," and the N2 produced is typically lost from soils and sediments to the atmosphere.
The balance between N fixation, denitrification, and dissimilatory NO3- reduction to N2 determines the net gain or loss of fixed nitrogen from a watershed. In contrast, other processes like assimilation and DNRA internally transform nitrogen between different biologically reactive forms within an ecosystem. Over past millennia, microbial nitrogen fixation was approximately balanced by microbial production of N2 gas in oceans and soils, resulting in a stable global C:N:P stoichiometry. However, this long-standing natural equilibrium is being profoundly disrupted by escalating rates of anthropogenic nitrogen fixation, altering ecosystem dynamics on a global scale.
Date added: 2025-11-17; views: 157;
