Cytokines. Cytokine Function in the Acute Phase Response
Cytokines. Within the last two decades and more particularly in the latter part of the 1980s, with the advent of molecular cloning techniques, there have been major advances in our understanding of the peptide mediators involved in the acute phase response. These peptides are synthesized and released from inflammatory and stromal cells and are variously referred to as lymphokines, monokines, interferons, growth factors, colony-stimulating factors, interleukins, or, collectively, cytokines, to indicate their primary role in cellular communication.
These peptides, many of which are glycosylated and have molecular masses in the 10- to 30-kD range, are active at the pico- and nanomolar levels. They interact with specific high-affinity (i.e., K9 = 10-9 to 10-12 M) receptors on the surface of various cells and elicit metabolic changes in the target cell. In this regard, and despite the fact that they are made and released by multiple dispersed cell types, they might be considered in the same way as other hormones (e.g., insulin or glucagon).
Thus, while we now know the identity of many cytokines, we know little about tissue distribution or bioavailability, nor do we know how each signal is received and deciphered by the individual cell, whether simultaneous or sequential. In addition, similar to the endocrine system, there are cascades of networks and cytokines. These too must be deciphered before the full role of the cytokines can be understood in the context of the acute phase response.
Initial work in the mid-1970s identified leukocyte- derived material, called endogenous pyrogen or leukocyte endogenous mediator, which could induce most aspects of the acute phase response when injected into experimental animals. Further work proved that the effects could be induced by a single cloned polypeptide, termed interleukin-1 (IL-1). This cytokine, which exists in two forms, IL-lα and IL-1β, is produced by various cell types, but primarily by activated mononuclear phagocytes. The cytokine is pleiotropic in action, causing many cells to undergo inflammatory changes, most notable of which is the induction of protaglandin synthesis and the secretion of further cytokines from the target cells.
A second cytokine that figures prominently in the acute phase response is tumor necrosis factor (TNF), which is identical to cachectin, the factor causing decreased lipoprotein lipase activity in endotoxin-treated animals. TNF has many of the same pleiotropic actions of IL-1, but in addition, can cause the wasting or cachectic response seen in chronic debilitating inflammatory diseases. Like IL-1, TNF causes the release of prostaglandins from various stromal cells and induces the synthesis and release of other cytokines from these cells. These two cytokines appear to be involved with the initiation of the acute phase response, causing some changes directly and others through secondary cytokine induction.
A third cytokine that features prominently in the acute phase response is IL-6. This peptide, first described as a В lymphocyte-stimulating factor and as a β-interferon, is the major factor eliciting the hepatic acute phase response. IL-6 is also released from activated mononuclear phagocytes, but more important is the fact that IL-6 is induced in fibroblasts and in epithelial and endothelial cells by IL-1 and TNF.
This secondary induction represents a potent recruitment and augmentation of the cytokine signal, since there are so many more stromal cells in the body than inflammatory cells. Like IL-1, IL-6 is also pleiotropic in its actions, causing fever as well as stimulating the immune response, in addition to its action on the liver.
Moreover, it is a potent thrombopoietin, causing the maturation of megakaryocytes and replenishing the platelet population, which can be used up in the early stages of the acute phase response. Leukemia inhibitory factor (LIF), first described as a T lymphocyte product, but known to be released by monocytes and stromal cells, was shown recently to be an additional cytokine that induces the hepatic acute phase response in a manner similar to that of IL-6.
Two other cytokines that have been identified recently have a major role in the accumulation and activation of neutrophils, monocytes, and lymphocytes. IL-8, or monocyte-derived neutrophil chemotactic factor, has potent in vitro and in vivo chemotactic activities for lymphocytes and neutrophils. IL-8 and its likely murine homolog macrophage inflammatory peptide 2 are members of a family of structurally related cytokines, which includes platelet factor 4 and β-thromboglobulin, both released from platelets upon aggregation, having chemotactic activity for neutrophils, monocytes, and fibroblasts.
Another chemoattractant cytokine, monocyte chemotactic and activating factor (MCAF), belongs to a second structurally related family of cytokines including the murine macrophage inflammatory peptide 1, which exhibits chemotactic and pyrogenic activities. These two cytokines, IL-8 and MCAF, are released by activated monocytes, and, like IL-6, they can be released from fibroblasts and endothelial cells upon stimulation with IL-1 and TNF.
A factor that previously received attention as a transforming factor for nonneoplastic cells and was recognized subsequently as a cytokine with immunomodulatory roles is transforming growth factor ß (TGF-β). Initially identified as coming from platelets, it is now recognized as a product of various hematopoietic cells, playing a role in the initiation of the acute phase response. Platelet aggregation leads to the degranulation and release of TGF-β, which has chemotactic activity for monocytes and enhances cytokine generation in these cells, including IL-1, TNF, and other growth factors.
Most of these cytokines and others with similar activity are released initially from activated mononuclear phagocytes (Table III). More recently, it has been recognized that the same cytokines are released from activated T lymphocytes, leukocytes, fibroblasts, and other stromal and mesenchymal cells.
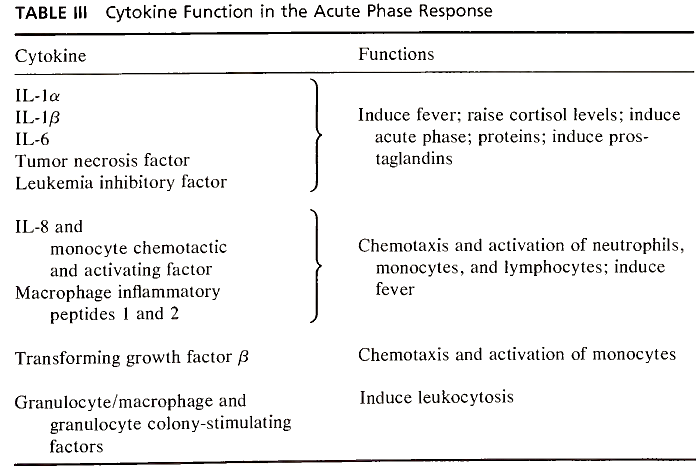
TABLE III. Cytokine Function in the Acute Phase Response
The biological activities of these cytokines and other related molecules are still being characterized, but we now recognize that there are families of these peptides which appear to be both functionally and structurally related. IL-1 and TNF appear to be distinct in that they can act directly to induce the acute phase response and indirectly by inducing stromal cell production of IL-6, IL-8, MCAF, and others.
Cells. The initial response within the vessels is the activation of platelets, with aggregation and degranulation resulting in clot formation and the release of vasoactive components such as serotonin and thromboxane A2, causing changes in vascular permeability and smooth muscle contraction. In addition, the release of ADP results in further platelet aggregation and activation of coagulation, while the release of platelet-derived growth factor results in the stimulation of cells (e.g., fibroblasts), giving rise to enhanced release of other cytokines, including IL-6 and IL-8. The release of TGF-ß by platelets can induce monocyte accumulation and activation, resulting in enhanced release of inflammatory cytokines, including IL-1 and TNF.
In turn, further activation and aggregation of platelets can be caused by the release of platelet-activating factor from mononuclear cells after the cells have been activated by trauma or infection, representing an amplification loop of cytokines and cells in the acute phase response. Since mononuclear cells also release IL-6, a potent thrombopoietin, one begins to see the complexity of the acute phase response, with the same cell releasing factors that both activate and utilize the platelet as well as factors that cause the replacement of these important cells.
Closely following the activation of platelets in the vessels is the accumulation of neutrophils within the involved tissue. We know that chemotactic molecules such as C5a and leukotriene B4 or formyl peptides can directly recruit leukocytes to the tissue. We also know that mononuclear cell-derived cytokines (e.g., IL-1 and TNF) can induce the expression of surface leukocyte adhesion molecules on endothelial cells as well as inducing the synthesis of neutrophil-activating cytokines (e.g., IL-8 and other growth factors) by stromal cells.
The combination of increased expression of intracellular adhesion molecules 1 and 2 and endothelial leukocyte adhesion molecule 1, coupled with the local production of colony-stimulating factors (granulocyte- and granulocyte/macrophage-CSF) and IL-8, can account for neutrophil margination (adherence to the vessel wall), extravasation, and chemotaxis, as well as leukocytosis from bone marrow activation and inflammatory cell maturation.
Thus, while it remains that a hallmark of acute inflammation is neutrophil accumulation, obviously platelet, mononuclear, and endothelial cell activation likely precede this accumulation, as does the local release of various cytokines with both microenvironmental and systemic effects. The multiple factors that can elicit neutrophil accumulation point to the importance of these cells in the survival of the host. Bacterial infection and monocyte activation lead to neutrophil chemotaxis via IL-8, while the same result would be seen through the release of platelet factor 4 by platelets in the acute phase associated with trauma.
The presence of mononuclear cell accumulation in the tissue has heretofore heralded the chronic stage of inflammation. However, work showing an early flux of monocytes into sites of inflammation, temporally equal to that of neutrophils, coupled with the knowledge that mononuclear phagocytes are the cells most likely releasing the early pulse of inflammatory cytokines, indicates that the tissue macrophages or the peripheral blood monocytes are the cells most likely to initiate the acute phase response.
Not only are the macrophages aptly placed within the tissues to encounter pathogens (e.g., the alveolar macrophage), but the release of cytokines such as MCAF and granulocyte/macrophage-CSF at local sites ensures the entry of monocytes into the tissues and the regeneration of adequate numbers of peripheral blood monocytes from the bone marrow. Thus, while the polymorphonuclear neutrophil is a prominent participant in the acute phase response, the mononuclear phagocyte might well be the important initiator of the response.
A number of other cells, normally classed as inflammatory, play significant roles in the initiation or promulgation of the acute phase response. Mast cells and basophils are potent sources of vasoactive agents. Upon activation with anaphylatoxins such as C3a and C5a, these cells release their granular contents.
These include histamine and serotonin, certain metabolites of arachidonic acid, including prostaglandin D2 and leukotrienes C4, D4 and E4, which are known as slow-reacting substances of anaphylaxis, because of their action in causing the prolonged contraction of smooth muscle. Moreover, the mast cell is a major source of plateletactivating factor, resulting in platelet activation and degranulation, as well as releasing a highly active chemotactic factor for eosinophils. The mast cell and basophil contribute directly to the altered vascular permeability seen in the acute phase response.
Date added: 2023-05-09; views: 750;
