Physiological Mechanisms in Acute Phase Response
Fever and Leukocytosis. Fever is one of the main physiological changes seen in the acute phase response and is considered a major host defense mechanism. Activation of T lymphocytes by IL-1 is significantly greater at elevated temperatures. T cell killing of tumor cells and the activity of cytotoxic T cells are also enhanced.
Moreover, fever reduces the replication of pathogenic organisms and leads to enhanced antibody formation by В cells. The temperature of the body is elevated by having the temperature set point in the hypothalamus raised above the normal range. The elevation is achieved through the local generation of arachidonate metabolites, particularly prostaglandin E2, within the hypothalamus.
A number of endogenous pyrogens, the most prominent of which is IL-1, can cause the induction of fever. Both forms of this cytokine induce a significant increase in body temperature, which is blocked by antipyretics such as aspirin. TNF is a pyrogenic cytokine with about the same potency as IL-1 and a-interferon, which has been administered clinically to humans, induces fever directly via the induction of brain prostaglandins.
Other recently described factors, such as the family of mouse macrophage inflammatory peptides 1 and 2, induce fever by both prostaglandin-dependent and -independent mechanisms. Obviously, activation of mononuclear phagocytes can result in the release of several of these endogenous pyrogens, and indirect activation of other cells by cytokine cascades can either augment these signals or deliver others in concert to the brain. The result is the febrile response caused by the same cytokines that initiate other aspects of the acute phase response.
Leukocytosis is the increase in total and relative numbers of circulating neutrophils, both mature and those undergoing maturation, or “band forms.“ The mechanism involves the action of CSFs, including granulocyte- and granulocyte/macrophageCSF, on the proliferation and differentiation of bone marrow precursors for neutrophils, resulting in the accelerated release of neutrophils from the bone marrow.
The precursors might circulate during maturation, giving rise to the appearance of band forms. Other forms of myeloid cell increases occur under certain circumstances (e.g., eosinophilia or mastocytosis, seen in some parasitic diseases). These too are caused by the release of different cytokines from inflammatory and stromal cells involved in the acute response to the invading organisms.
Acute Phase Proteins. One of the more striking systemic changes that occur during the acute phase response is a rapid dramatic alteration in the plasma concentration of a series of proteins, commonly called acute phase proteins, which are primarily made by the liver. The changes appear to be independent of the cause of inflammation, whether it is trauma or burns; viral, bacterial, or parasitic infection; or tissue necrosis.
The hepatic response is the most well studied and well understood aspect of the acute phase response. The fact that this reaction has been strongly conserved through evolution in vertebrate species indicate that this response plays a major and primary role in systemic homeostasis.
Cytokines involved in the other aspects of the acute phase response are also those involved with the alteration of liver metabolism. In considering the cytokines which should be classed as hepatocyte-stimulating cytokines, we restrict ourselves to considering those molecules which interact specifically with hepatocytes through their own receptors and induce acute phase protein gene changes similar to those seen in vivo during inflammation.
The many acute phase proteins derived from the liver differ in their physicochemical properties and functional activities. Most of them are glycoproteins, and the kinetics and magnitude of plasma concentration changes are characteristic for each protein. An examination of their known physiological roles (Table IV) indicates that acute phase proteins can both mediate and be consumed by the inflammatory process. Two proteins in humans—C-reactive protein and serum amyloid A protein—rise up to several hundredfold from undetectable levels, while others, including fibrinogen, haptoglobin, and α1 acid glycoprotein, rise to four to five times the normal level. Other proteins, including albumin, transferrin, and a- and β-lipoproteins, decrease in circulation during the acute phase response.
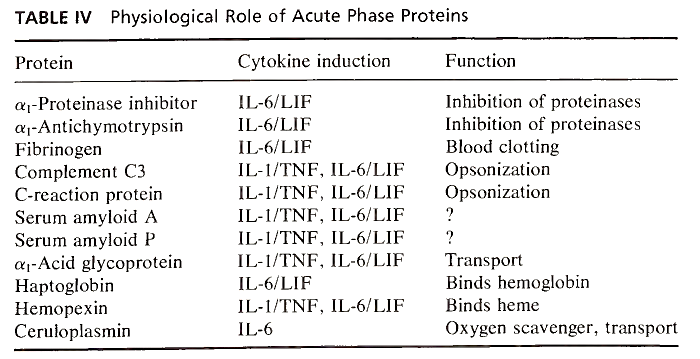
TABLE IV. Physiological Role of Acute Phase Proteins
The physiological role of some acute phase proteins is well recognized. Complement C3 and C- reactive protein opsonize bacteria, immune complexes, and foreign particles, while fibrinogen is involved in blood coagulation and is consumed in the homeostatic response to trauma and therefore must be rapidly replaced. Other acute phase proteins play a major role as inhibitors of various serine and cysteine proteinases, most notably those released from activated neutrophils. α1-Proteinase inhibitor (α1-antitrypsin) specifically inhibits neutrophil elastase, while α1-antichymotrypsin inhibits leukocyte cathepsin G.
The general nature of the acute phase protein response is that of controlling proteolytic activity and inhibiting the trauma and destruction associated with inflammation. In addition to mediating homeostatic responses, a number of the proteinase inhibitors have also been shown to modulate immune reactions, at least in vitro. α1 - Proteinase inhibitor and α1-chymotrypsin modify the activity of natural killer cells and antibody-dependent cell-mediated cytotoxicity. Complexes between inhibitors and specific proteinases can suppress macrophage activation and la antigen expression.
One of the major acute phase proteins in humans, α1-acid glycoprotein, previously called orosomucoid, might also be an immunomodulatory molecule, although the data supporting this contention are less solid than for the proteinase inhibitors. C- reactive protein, while appearing to have immunomodulatory activity, as well as behaving as a scavenger and opsonizing factor for chromatin fragments released from damaged cells, is one of the acute phase proteins whose role and main physiological function remain undescribed.
By examining the overall acute phase protein response, it appears that the role of this response is to control the random destruction associated with inflammation and to mediate tissue repair and the return to normal function. In addition, there might be involvement in mediating the subsequent immune response, and, as such, the proteins play a broad protective role during the early stages of the host response to invasion prior to initiation of the specific immune response.
The great majority of the acute phase proteins are primarily synthesized by the hepatocyte. However, there is recent evidence that a number of these proteins, particularly the antiproteinases, might be synthesized by mononuclear cells. The control of synthesis within this extrahepatic population is under the same cytokine involvement as the liver, and while the level of synthesis is much lower than that of the hepatocyte, this mononuclear cell synthesis could be important in modifying the microenvironment around cells involved in activation or immune regulation.
Initially, proinflammatory cytokines such as IL-1 and TNF were thought to regulate the hepatic response since, when one injected the purified or recombinant material into an animal, plasma changes in the acute phase proteins were seen. However, with the availability of cloned and purified material and in vitro hepatocyte culture systems, other cytokines have been identified which appear to be the major regulators of acute phase protein gene expression.
Concluding Remarks
Subsequent to invasion of the body by infectious organisms or activation by trauma, there is a series of cascading events involving cells, both inflammatory and stromal cells, and a family of acute phase cytokine hormones with multipotent and overlapping biological activities (Fig. 1). Within the tissue undergoing challenge, the cells and the cytokines interact along with vasoactive mediators to allow vessel permeability and the influx of inflammatory cells.
The same signals act systemically at various target tissues, including the hypothalamus, to mediate fever; the bone marrow, to mediate leukocytosis; and the liver, to mediate acute phase protein synthesis. Interruption of the acute phase response pharmacologically can occur at various stages. Drugs such as aspirin and indomethacin can decrease the synthesis of prostaglandins and interrupt the febrile response. More potent drugs such as steroids have a broader spectrum of activity, inhibiting the activation of cells and the generation of the various cytokines.
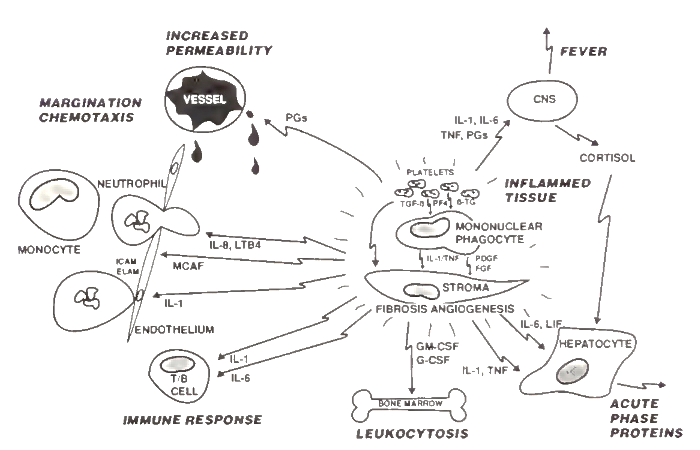
FIGURE 1. Cell and mediator interaction in the acute phase response. Mediators released from the local inflamed tissue interact with multiple tissues and organs to induce the acute phase response. Platelets—through mediators such as transforming growth factor ß (TGF-β), platelet factor 4 (PF4), and β-thro- moglobulin (β-TG)—and mononuclear phagocytes, through cytokines interleukin-1 (IL-1), tumor necrosis factor (TNF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF)—involve and activate the local stromal cells in the inflamed tissue.
The tissue-derived mediators effect other systemic responses. Prostaglandins (PGs) mediate vascular permeability. IL-1, TNF, IL-6, and PGs affect the brain and induce fever as well as the synthesis of corticosteroids. IL-1, IL-6, TNF, and leukemia inhibitory factor (LIF) along with corticol mediate the hepatic acute phase response. Granulocyte/macrophage- and granulocyte-colony stimulating factors (GM- and G-CSF, respectively) induce leukocytosis. IL-1 and IL-6 induce В and T cell activation and the immune response.
IL-1 activates endothelial cells and causes the expression of intracellular adhesion molecules (ІСЛМ) and endothelial leukocyte adhesion molecules (ELAM), and IL-8, leukotriene B4 (LTB4), and monocyte chemotactic and activating factor (MCAF) cause leukocyte margination and chemotaxis. CNS, Central nervous system
It is clear that the acute phase response is an early protective response, controlled by multiple overlapping signals, which ensure that the response is swift and complete, since it is this early response that provides us with our main protection before the more sophisticated and specific immune response can play a role.
Bibliography: Beutler, В., and Cerami, A. (1988). Tumor necrosis, cachexia, shock and inflammation: A common mediator. Annu. Rev. Biochem. 57, 505.
Dinarello, C. A. (1986). Interleukin-1: Amino acid sequences, multiple biological activities and comparison with tumor necrosis factor (cachectin). Year Immunol. 2, 68.
Dinarello, C. A., Cannon, J. G., and Wolff, S. M. (1988). New concepts on the pathogenesis of fever. Rev. Infect. Dis. 10, 168, 34-64.
Fantone, J. C. and Ward, P. A. (1988). Inflammation. In “Pathology” (E. Rubin and J. L. Färber, eds.) Lippincott, Philadelphia, Pennsylvania.
Gauldie, J., and Baumann, H. (1990). Cytokines and acute phase protein expression. In “Cytokines in Inflammation” (E. H. Kimball, ed.). Telford, Caldwell, NJ.
Koj, A. (1985). Definition and classification of acute- phase proteins. In “The Acute-Phase Response to Injury and Infection” (A. H. Gordon and A. Koj, eds.). pp. 139-144. Elsevier, Amsterdam.
Mantovani, A., and Dejana, E. (1989). Cytokines as communication signals between leukocytes and endothelial cells. Immunol. Today 10, 370.
Matsushima, K., and Oppenheim, J. J. (1989). Interleukin 8 and MCAF: Inflammatory cytokines inducible by IL 1 and TNF. Cytokine 1, 2.
Wahl, S. M., McCartney-Francis, N., and Mergenhagen, S. E. (1989). Inflammatory and immunomodulatory roles of TGF-/3. Immunol. Today 10, 258.
Wolpe, S. D., and Cerami, A. (1989). Macrophage inflammatory proteins 1 and 2: Members of a novel superfamily of cytokines. FASEB J. 3, 2565.
Date added: 2023-05-09; views: 724;
