Electromagnetic Radiation Emission Mechanisms
Having looked at thermal emission, it is now important to consider the other types of emission mechanism for electromagnetic radiation. Considering bound-bound transitions, it becomes natural to wonder if it is possible for electrons to completely escape the influence of their atomic nuclei simply by absorbing photons of sufficiently high energy. This is a process known as photoionisation and is defined as a bound-free transition.
The energy required to ionise a hydrogen atom, if its electron is in the lowest energy or ground state, is 13.6eV. (An electron volt is a measure of the energy gained by an electron if it is accelerated through a potential difference of 1 volt.) Photons containing this amount of energy or more can be absorbed and used to eject the electron from the atom, leaving an atomic nucleus with a net positive charge known as a positive ion. Energy over and above the 13.6eV needed for ionisation will be converted into kinetic energy by the free electron.
As the photon energy increases past the ionisation limit, however, the probability that the photon will be absorbed decreases and so absorption bands are created in the spectrum which have a sharp discontinuity corresponding to the energy of ionisation (see Fig. 2. 6). Several such bands are possible within the same spectrum because ionisation can take place from electrons in any energy level. A gas cloud which is largely composed of positive ions and electrons is known as a plasma.
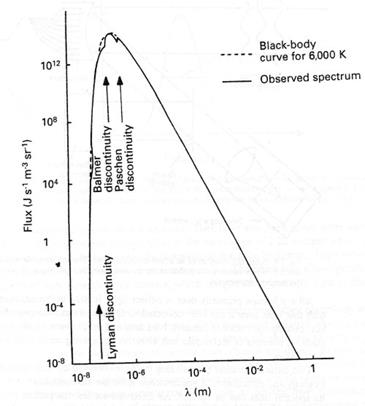
Fig. 2. 6. Schematic continuous spectrum of a solar-type (G-type) star, showing ionisation bands. (Adapted from Kitchin, C., Stars, Nebulae and the Interstellar Medium, Adam Hilger, 1987.)
Conversely, free electrons can be captured by positive ions and their energy given out as photons. This is the process of recombination and is known as a free-bound transition.
The final type of emission mechanism to be considered is a range of mechanisms which could be classed as free-free transitions. In all of these interactions the electrons are unbound to atomic nuclei and remain that way, even after the interaction which produces the radiation. The most obvious of the free-free transitions is known as thermal bremsstrahlung. This occurs when a free electron is decelerated by an interaction with another charged particle.
The other particle may be a positive ion (i.e. an ionised atomic nucleus) or another free electron. Whatever the precise situation, the energy lost by the electron in the interaction is released as a single photon of radiation. A thermal bremsstrahlung spectrum is continuous but of a very different shape from a black body curve. It is the characteristic emission of a tenuous plasma.
Magnetobremsstrahlung is also possible but usually goes by the name of synchrotron radiation. This emission mechanism involves particles travelling at relativistic velocities in circular trajectories through a magnetic field. This causes the electron to lose energy by giving out electromagnetic radiation. Unlike other emission mechanisms the radiation produced in this way is highly polarised. This means that, instead of being randomly orientated, the electric vectors of the photons are consistently placed in the same direction.
The Compton effect occurs when a high energy photon interacts with a lower energy electron. It is convenient to imagine that the photon is scattered and knocked into a lower energy state, whilst the electron is boosted to higher energies.
The inverse process occurs when a high energy electron interacts with a low energy photon and boosts its energy. Described in this way it sounds rather similar to synchrotron radiation, with a photon field replacing the magnetic field. Although we have referred to these effects taking place between electrons and photons, which will apply in the majority of astrophysical cases, the electrons could be replaced by other particles.
The wavelength shift, Δλ, suffered by a photon in the inverse Compton effect is given by the equation:

where ϕ is the scattering angle of the photon and λc is the Compton wavelength of the particle undertaking the photon interaction. The Compton wavelength is the wavelength a photon would possess if it carried the rest energy of the particle; hence it is defined:

where m0 is the mass of the particle involved in the scattering.
Date added: 2023-09-14; views: 722;
