Early Oogenesis. Gonadotrophins and the Ovary
Unlike the male, the female germ cell (oogonia) undergoes mitotic proliferation in the ovary prior to the time of birth. All of these oogonia possess 46 chromosomes. From about the third month of gestation, increasing numbers of oogonia start to enter their first meiotic division, thereby becoming primary oocytes with a chromosome complement of 23. By the time of birth, or soon after, all female germ cells are primary oocytes.
At all stages of follicle development, however, degeneration, or atresia, occurs. In the human female, about 99% of oocytes are lost in this way. The consequence of atresia is that the number of oocytes is gradually reduced from birth until the time of menopause in women, when very few oocytes can be detected in histological sections of the ovary. Changes in germ cell numbers during the life of a human female are illustrated in Fig. 2.
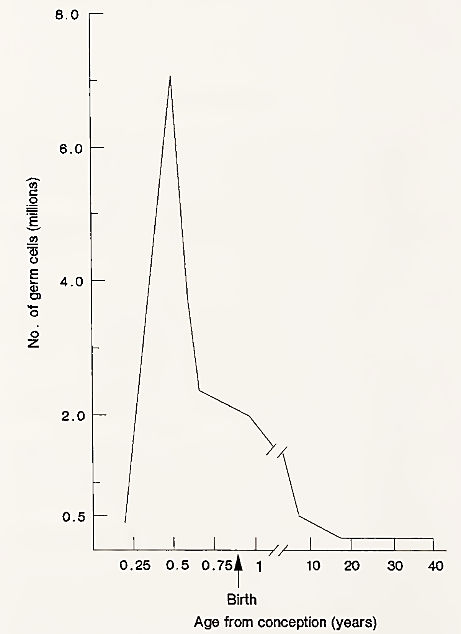
FIGURE 2. Total number of germ cells in the human female during life. Peak numbers occur before birth and then continuously decline until menopause. [From T. G. Baker (1971). Am. J. Obstet. Gynecol. 110, 746.]
Soon after formation, the primary oocyte becomes surrounded by a single layer of flattened epithelial (granulosa) cells. This group of cells constitutes the primordial follicle. Further meiotic division of the primary oocyte is halted at the dictyotene stage of prophase and it may remain in this state for up to 50 years. It is not known why the oocytes are stored in this protracted meiotic state.
The control of the next stage of folliculogenesis is not well understood but seems to be independent of gonadotrophin support. The oocyte undergoes its major growth phase marked by massive synthetic activity and morphological changes. The granulosa cells become cuboid in shape and increase in number, forming four or five layers around the oocyte, a preantral follicle.
However, during the next phase of follicular development, which occurs from puberty onward, growth is dependent on continuous secretion of hormones from the anterior pituitary. At the beginning of each menstrual cycle, a group, or cohort, of follicles enters the gonadotrophin-dependent phase of growth. In the next section, the role of these hormones in the human female will be discussed.
Gonadotrophins and the Ovary.Gonadotrophin-releasing hormone (GnRH) is secreted episodically into the portal blood system linking the hypothalamus to the anterior pituitary. GnRH release is affected by steroid feedback from the ovary as well as external environmental cues. GnRH stimulates specialized cells in the pituitary (the gonadotrope cells) to secrete the gonadotrophins—luteinizing hormone (LH) and follicle-stimulating hormone (FSH). [See Hypothalamus; Pituitary.]
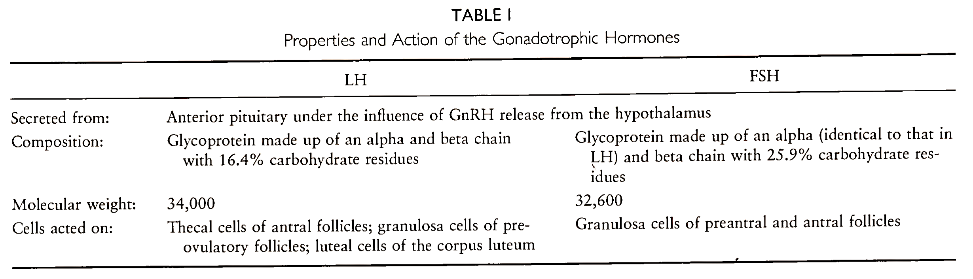
The functioning of the adult ovary is dependent on the secretion of these gonadotrophic hormones. These interrelationships are diagrammatically represented in Fig. 3. The main properties of the two gonadotrophins, LH and FSH, and their action in the human female are shown in Table I.
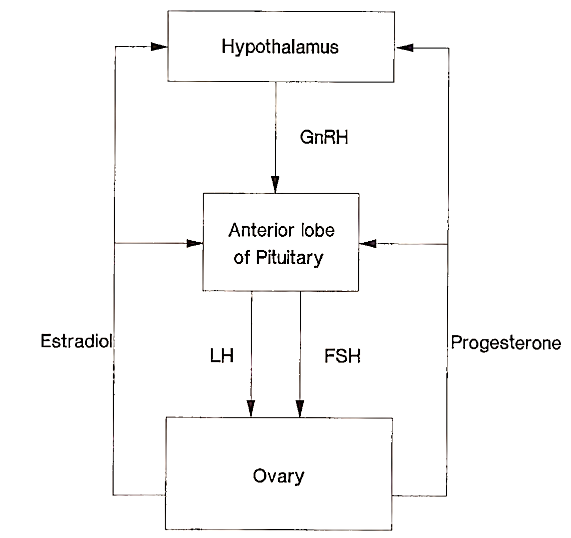
FIGURE 3. Hormone secretion between the hypothalamus, pituitary, and ovary. Estradiol and progesterone are the most important ovarian steroids that modulate gonadotrophin secretion in the follicular and luteal phases, respectively. Depending on the stage of the menstrual cycle, these steroids can have both positive and negative feedback effects upon gonadotrophin secretion (see text)
The effect of LH and FSH on the ovary is to promote the synthesis of estradiol-17b, the predominant estrogen secreted by the ovary. In turn, estradiol, acting on the hypothalamic-pituitary axis, modifies LH and FSH secretion. During the luteal phase progesterone is the predominant ovarian steroid that modulates gonadotrophin secretion.
Before discussing in detail follicular growth, it is necessary to first consider how estradiol is synthesized by the ovary. Estradiol production is a cooperative venture involving both the theca interna and the granulosa cells of the follicle.
Date added: 2022-12-11; views: 765;
