The Two-Cell Theory of Follicular Steroidogenesis
The requirement for cell cooperation in the production of estradiol-17B was first demonstrated by B. Falck in 1959. In a series of classic experiments, he showed that estrogen formation by the rat follicle depends on the joint action of the theca interna and the granulosa cell layers.
In 1962, R. Short first proposed a two-cell theory to explain follicular steroidogenesis. This hypothesis was subsequently modified, as later experiments showed that, although thecal and granulosa cells can independently synthesize estrogens, the yield is greatly increased if both cell types are incubated together. This interaction of two cell types, independently stimulated by the two gonadotrophins, LH and FSH, led to the final realization of a “two-cell type, two-gonadotrophin” theory for estradiol synthesis in the ovary, as illustrated in Fig. 4.
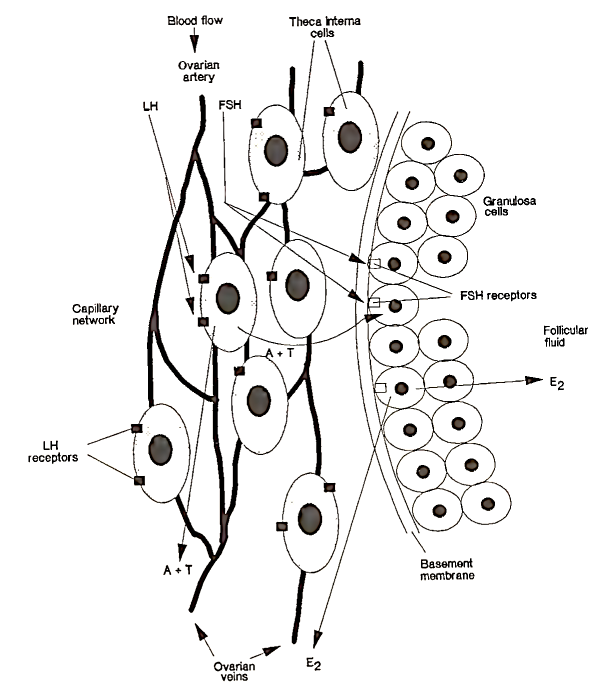
FIGURE 4. Schematic representation showing the action of the gonadotrophins on the follicle cells and the synthesis of estradiol. LH attaches to specific receptors on theca interna cells. Under LH stimulation, these cells produce androgens (androstenedione, A; testosterone, T). These steroids are secreted into the blood or pass through the basement membrane of the follicle and enter the granulosa cells. FSH interacts with receptors on the granulosa cells and activates the aromatase enzyme system, which converts androgens to estradiol (E2). E2 is either secreted out of the follicle and into the bloodstream or concentrated in follicular fluid. (From Baird, 1984.)
Although this theory requires the involvement of both gonadotrophins for normal follicular steroidogenesis to occur, recent data suggest that normal folliculogenesis can occur independently of LH secretion. In these experiments, follicular development was induced and sustained by administering recombinant human FSH (r-hFSH), however, estradiol secretion was negligible.
The principal androgens, androstenedione and testosterone, are produced by the thecal cells, which are stimulated by LH. The androgens pass into the blood or are transported across the basement membrane of the follicle into the granulosa cell, where they are converted into estradiol by the aromatase enzyme stimulated by FSH. Estradiol is then secreted back into the blood or accumulates in the follicular fluid, keeping the intrafollicular environment highly estrogenic. For a mature follicle, the concentration of estradiol in the follicular fluid can be 1000 times, that circulating in the blood. This could further potentiate LH and FSH action, as estradiol has been shown to increase the sensitivity of the follicle to gonadotrophin stimulation.
Evidence from numerous in vitro experiments has demonstrated the importance of paracrine factors [inhibin, activin, insulin-like growth factor-I and -II (IGF-I and IGF-II)] in the control of ovarian steroidogenesis. Of particular importance is inhibin, which is secreted from FSH-stimulated granulosa cells. Inhibin has been shown to promote FH-stimulated androgen synthesis in vitro by human thecal cells. These findings have important clinical implications as they help to explain why, in situations where there are very low FH levels, normal follicular growth and ovarian steroidogenesis can be driven by exogenous FSH administration alone.
The importance of this two-cell type-two-gonadotrophin interaction for normal follicular development is aptly illustrated in the clinical condition known as polycystic ovarian disease (PCOD). Although the etiology of this condition is complex, it is sufficient to say that in 80% of subjects FH secretion is higher than normal, which is a contributing factor to excess production of androgens by the thecal cells. Patients with PCOD can suffer with chronic anovulation and are more likely to be obese. The ovaries of these women contain numerous antral follicles that are androgenic rather than estrogenic. This can be rectified by the administration of FSH, which initiates normal follicular growth and estradiol production.
Date added: 2022-12-11; views: 703;
