Clocks, Atomic. Atomic Hydrogen Maser
Atomic clocks rely on the precise timing of the oscillation frequency of certain atoms which are energized or excited to another energy state. The near immutability of the energy levels in the atomic structure of certain paramagnetic elements provides atomic clocks with the inherent reproducibility and long-term stability previously lacking in material standards such as quartz clocks or the Earth’s rotation period.
Some ten thousand (commercial) atomic clocks are now in operation throughout the world ensuring essential control and synchronization in a wide range of applications in science and technology, such as in the NAVSTAR satellites used in the global positioning system (GPS).
The readings of a subset of more than 250 clocks maintained in about 60 national institutions are combined with those of about 12 laboratory clocks to form International Atomic Time (TAI), the basic time scale for science and technology and whose derivative, Coordinated Universal Time (UTC), which differs from TAI by an integral number of seconds, provides the atomic equivalent of mean solar time for general use worldwide.
The principle underlying atomic clocks is that when individual atoms of the same element absorb or emit energy by electrons moving from one energy level to another, the radiation produced by individual atoms has exactly the same (quantized) frequency. These resonant frequencies, stable in time and space, are which makes the atoms perfect timepieces. The oscillations are changed by environmental factors such as humidity that would decrease the accuracy of normal clocks.
The main sources of atomic timekeeping are cesium and rubidium clocks with a smaller, but significant, contribution from active hydrogen maser clocks. In the atoms of all three elements the ground state (lowest-energy state) is split into two hyperfine levels by the interaction between the spin (and consequent magnetic moment) of the single valence electron and the spin of the atomic nucleus.
The energy difference, ΔE, between these two closely separated states determines a resonant or characteristic frequency v given by v = ΔE/h, where h is Planck’s constant. Transition from the top to the lower state causes emission of a photon of that frequency. The states are nearly equally populated and the necessary unbalance; that is, alteration of the distribution of atoms in a given state, can be achieved by either magnetic state selection involving spatial separation of the atoms (selecting only those in one hyperfine state) or optical pumping using differential transfer to excited states.
The magnetic approach was first employed in the 1920s when Otto Stern and Walther Gerlach in Germany made use of high-gradient magnetic fields to deviate a beam of (silver) atoms, each hyperfine state being oppositely deflected, confirming space quantitization. The method was subsequently improved in the late 1930s by Isodor Rabi at Columbia University who added a radiofrequency field to stimulate transitions between the hyperfine states and thus brought together the elements of a magnetic resonance machine that could function as a frequency standard. Those atoms that are stimulated to change state emit light.
The frequency of the radio-frequency field is varied, and eventually, a frequency is achieved that alters the states of most of the atoms and maximizes their fluorescence. A counter counts the radio-transmitter pulses, which gives a precise frequency standard that can be used to define a time standard.
In 1949 Rabi’s colleague Polykarp Kusch produced a basic design concept for a cesium atomic clock, his proposals incorporating a Ramsey cavity, following the discovery in the same year by Norman Ramsey at Harvard University that coherent excitation applied at the beginning and end of an interval of beam travel was equivalent to atom-field interaction over the whole interval, thereby reducing substantially the width of the resonance line.
In the following years work began at several laboratories on cesium beam resonators based on the Kurch design. In the U.S. Harold Lyons at the (then) National Bureau of Standards achieved a linewidth of 300 hertz in 1952 with Ramsey excitation. At the Massachusetts Institute of Technology (MIT) Jerrold Zacharias developed a compact, transportable clock in 1954 which would form the basis for the first commercial production in late 1956.
Meanwhile, at the U.K. National Physical Laboratory (NPL), Louis Essen and John Parry, largely following the Kurch recipe, brought a cesium beam standard into operation in June 1955. This date represents the start of atomic timekeeping for, unlike the U.S. developments the NPL resonator was linked to an existing quartz clock ensemble and the resulting atomic clock could be routinely compared with astronomical time. The calibration of the cesium frequency in terms of Ephemeris Time over a three-year period led in 1967 to the formal redefinition of the second as 9,192,631,770 periods of the cesium-133 hyperfine transition frequency.
The present uncertainty in the realization of the second by laboratory standards making use of magnetic selection is about part in 1014, equivalent to nearly 1 nanosecond (10-9 seconds) per day while the commercial clock contributing to TAI display stabilities of the same order. The essential features of a commercial resonator are shown in Figure 4.
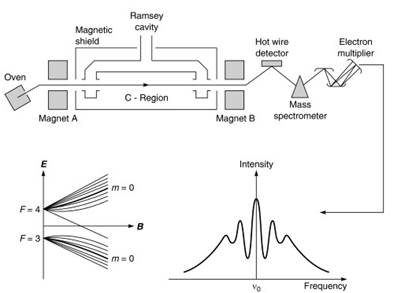
Figure 4. Features of a commercial resonator. [Source: Audoin, C. and Vanier, J. Atomic frequency standards and clocks, J. Phy. E: Sci. Instrum., 9, 697-720, 1976.]
Atoms traveling at about 200 meters per second are selected by magnet A in one of the hyperfine levels and make the transition (F = 4, m = 0)(F = 3, m = 0), the Zeeman substates m = 0 having least dependence on the low magnetic field in the C region. Thereafter, the atoms are directed by magnet B to a surface ionization detector, evaporating as positive ions which provide the servo-output to drive the frequency of the exciting oscillator to the peak of the Ramsey pattern.
Alfred Kastler in 1950 proposed a scheme of optical pumping which would be fully exploited only many years later with the advent of diode lasers. However, the partial overlap in the spectra of the rubidium isotopes 85Rb and 85Rb enabled optically pumped rubidium gas cell frequency standards and clocks to be realized in the late 1950s. The main features are shown in Figure 5.
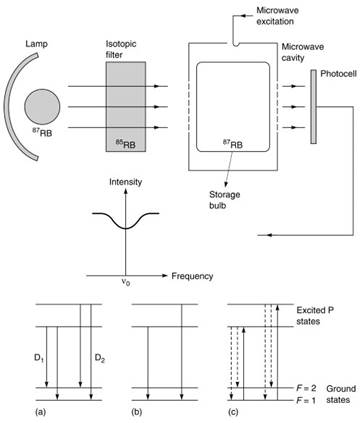
Figure 5. Features of optically pumped rubidium gas cell frequency clocks. [Source: Audoin, C. and Vanier, J. Atomic frequency standards and clocks, J. Phy. E: Sci. Instrum., 9, 697-720, 1976.]
The two D lines at wavelengths of 780 and 795 nanometers in the 87Rb lamp in (a) are differentially absorbed in the 85Rb filter cell (b), the residual light allowing transitions only from the F=1 hyperfine level (c) in the vapor cell, thus depopulating the level and making the cell more transparent.
Microwave excitation at the hyperfine frequency equalizes the hyperfine populations again, resulting in increased light absorption and a consequent fall in detector output, thereby providing a control signal to the microwave source. A buffer gas in the cell shield the rubidium atoms from depolarizing contact with the cell walls and largely eliminated Doppler broadening of the resonance line.
In the 1950s Zacharias at the Massachusetts Institute of Technology had constructed a vertical cesium resonator with the intention of achieving a long Ramsey interval using slow atoms interrogated as they rose and fell under gravity through a single cavity.
He was not successful using thermal atoms but the ‘‘Zacharias fountain’’ was finally realized by Andre Clairon and his colleagues at the Paris Observatory in 1993, making use of atoms cooled to a few microkelvin, state-selected by optical pumping and then periodically projected upwards at speeds of only a few meters per second to give a resonance width of about 1 hertz. Several fountain clocks are now in operation and contributing to TAI with an uncertainty of about 1 part in 1015 equivalent to 100 picoseconds (10-12 seconds) per day.
The clocks so far considered have been passive devices requiring external excitation. The atomic hydrogen maser, shown in outline in Figure 6, first operated in Ramsey’s laboratory at Harvard in 1960. It produces a signal at about 1420 megahertz albeit at the low level of around 10-12 watt.
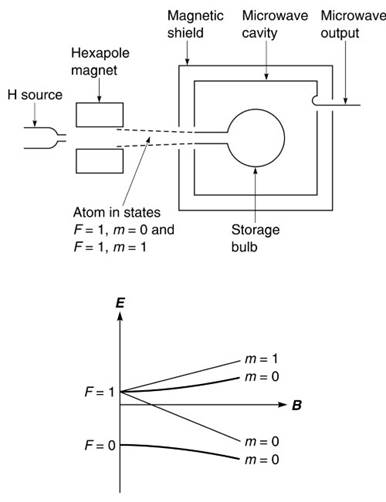
Figure 6. Atomic hydrogen maser. [Source: Audoin, C. and Vanier, J. Atomic frequency standards and clocks, J. Phy. E: Sci. Instrum., 9, 697-720, 1976]
Magnetic state selection focuses atoms in the F = 1, m = 0.1 states into the bulb immersed in a low-loss cavity resonator. A film of Teflon applied to the wall of the bulb allows atoms to make thousands of contacts with the wall while giving up energy to the cavity through the (F = 1, m = 0) — (F = 0, m = 0) transition. If this energy exceeds the cavity loss self-sustaining oscillations will result. The hydrogen maser has a short-term stability approaching 1 part in 1016 and about 50 hydrogen maser clocks now contribute to TAI.
Date added: 2023-10-03; views: 714;
