Water Molecules: Compositions, Structure, and Fundamental Interactions
Recognizing that the unique, life-giving properties of water result from the mere chemical combination of one oxygen atom and two hydrogen atoms illustrates the efficiency and creativity of nature. It is seen that essentially every detail of the water molecule’s structure has an important bearing on these resultant physical and chemical properties. Therefore, we begin our presentation with a review of the H2O molecule’s structure.
H and O Atoms: Atomic Building Blocks of Water. As the formula H2O shows, single water molecules (and any quantity of the substance, from molecular clusters to drops and upward to oceanic volumes) are composed of hydrogen and oxygen atoms in an atomic (or molar) ratio of two to one. Hydrogen and oxygen atoms participate in one and two covalent bonding interactions, respectively, to complete their valence electron shells and attain relative stability.
The simple proportion in which these atoms combine is a natural consequence of the positions of these elements in the periodic table and their ground-state electronic configurations. In the following sections, we will also see that the structures and key features of H2O molecules also fundamentally arise based on the natures of the constituent atoms.
Polar Covalent Bonding. As tabulated in Pauling’s seminal book, the electronegativity (EN) values of O and H atoms are 3.44 and 2.20, respectively. Accordingly, the atoms have an EN difference of 1.24 across their bond. According to Pauling, when bonded atoms have a difference in EN of this intermediate size, a polar covalent bond exists between them.
The bonding electrons are not equally shared in such a bond. There is a somewhat greater probability for electron density in the region of the more electronegative oxygen atom and a corresponding deficiency of electrons around the relatively electropositive hydrogen. This slight imbalance of charge throughout the molecule is indicated by the partial charge symbols δ+ and δ- on the molecular structure shown in Figure 1a. As a result of the imbalanced distribution of charge, the molecule is said to have polar bonds.
Bent Molecular Shape. According to valence shell electron pair repulsion (VSEPR) theory, the structure of the water molecule is based on the tetrahedral electron domain geometry that exists around the central oxygen atom. The molecular geometry is reduced from tetrahedral to bent because only two of the vertices of the oxygen-centered tetrahedron are occupied by H atoms, with the other two vertices being occupied by lone electron pairs.
Although every bond angle in a perfectly tetrahedral molecule (such as methane, CH4) is 109.5°, the actual H-O-H bond angle in the water molecule is 104.5°. This difference is a result of the fact that there are greater repulsive forces between the lone electron pairs in the valence shell around oxygen than there are between the electron pairs of the O-H bonds.
Based on the fact that the polarized H-O bonds in H2O are not oriented 180° relative to each other, the individual bond dipole moments do not cancel, and the molecule has net polarity. It is useful to compare carbon dioxide (CO2) and H2O molecules in this regard. Each of these triatomic molecules has a central atom that is bonded to two surrounding outer atoms via polar covalent bonds.
However, in the linear CO2 molecule, whose structure is dictated by the presence of only two electron pair domains, the dipole moments of the C=O bonds are oriented 180° relative to each other, which leads to complete cancellation of the bond dipoles and results in a completely nonpolar molecule. In H2O, however, the molecule has a nonlinear geometry, and the O-H bond polarities do not fully cancel each other. The vector sum of the bond polarities leads to an overall net polarity.
Hydrogen Bonding in Water. Figure 1b illustrates a pair of interacting H2O molecules. As the molecules approach, they lower their energy by adopting an orientation in which the partially negatively charged region (i.e. O end) of one molecule approaches the partially positively charged region (i.e. H atom end) of the other molecule.
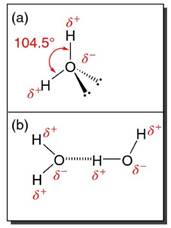
Figure 1. (a) Structure of single H2O molecule, indicating bond polarity and bent molecular geometry. (b) A pair of H2O molecules interacting via a hydrogen bonding interaction
The proximity with which the molecules approach enables the overlap of molecular orbitals; one of the oxygen-centered, filled lone electron pair orbitals on one molecule interacts with the unfilled antibonding (σ*2s) orbital of the neighboring molecule. This interaction contributes to the molecular pair’s stability. Due to this orbital interaction, there is a slight increase in the O-H bond length in the molecule whose σ*2s, antibonding orbital receives electron density.
Simultaneously, the H atom moves toward the electron density donating O atom of the molecule with which it interacts. In theoretical analyses of H-bonding in water, the bond that forms between the interacting molecules is considered to have some covalent character, and the strength has been estimated to be 3.6 kCal mol-1. The intermolecular interaction between H2O molecules can be viewed as an example of a dipole-dipole interaction.
However, this particular type of dipole-dipole interaction, because of its special properties, partial covalent character, and unusually high strength, is given a special name: hydrogen bonding. Hydrogen bonding can occur in any substance in which an H atom is bonded to a relatively electronegative and compact (second row) atom (N, O, or F). The significance of H-bonding in water to water’s acid-base chemistry will be discussed below.
Date added: 2023-10-03; views: 942;
