Cryogenics, Liquefaction of Gases
The modern air liquefaction industry generates annual sales in the tens of billions of dollars through production of liquefied oxygen, nitrogen, argon, and specialty gases. Following liquefaction, air is fractionally distilled to separate the various components. The cryogenic liquids are then stored and transported in specially insulated vessels and shipped to thousands of customers for a variety of applications.
Oxygen is utilized in the manufacture of steel, other metals, and glass, in the pulp and paper industry, in chemical processes, and for life support in medical applications. Nitrogen finds application in chemical processes such as the production of ammonia, in rapid-freeze food processing, in biomedical systems that preserve blood, tissue or semen, and in ecological recycling of car tires. Argon provides an inert atmosphere for the manufacturing of steel and aluminum, for welding, and for fabricating electronics components. It is also used for lighting, as are neon, krypton, and xenon.
The two coldest cryogenic liquids, hydrogen and helium, are utilized in various industries, hydrogen finding uses in chemical and food processing, pharmaceuticals, fuel propellant for spacecraft, and in emerging fuel cell technologies. Helium is used extensively in the aerospace industry, as an inert atmosphere in metal processing, for leak detection in HVAC and vacuum systems, and as a coolant for superconducting magnets and low temperature research.
Producing and using the liquid cryogens has been a leapfrog process in which the possibility of obtaining the pure gases has spawned new applications, with the resulting applications motivating new possibilities in the cryogenics industry. With the exception of utilizing oxygen for making steel, and nitrogen for agricultural fertilizer, none of the applications cited above existed in 1900. It was only in 1895 that the patents enabling commercial production of liquid air, oxygen, and nitrogen, were issued to Carl von Linde in Germany and William Hampson in Great Britain.
Figure 25 schematically diagrams the mechanical components and flow of gas through the Linde-Hampson air liquefier. As the air is compressed it rejects heat to its surroundings. Additional cooling of the pressurized air occurs in the counter-flow heat exchanger from the colder return-gas. Exiting the heat exchanger, the pressurized air is expanded through a valve resulting in a drop in pressure and temperature, to the extent that some of the air exits the valve in liquid form.
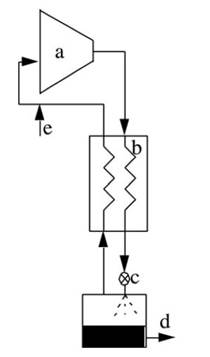
Figure 25. Linde-Hampson air liquefier: (a) compressor; (b) counterflow heat exchanger; (c) expansion valve; (d) liquid air outlet; and (e) make-up air inlet
The remaining cold gas returns up through the counterflow heat exchanger. Being warmed by the incoming pressurized air, it returns to the suction side of the compressor near room temperature. Adding the same mass in gaseous form at the inlet of the compressor then compensates for the amount of liquid that is extracted from the machine.
In 1902 Georges Claude improved the design of air liquefiers by replacing the expansion valve with an expansion piston, producing liquid more efficiently because the gas is cooled by ‘‘doing work’’ as it pushes against the piston during the pressure drop. In the same year, and because of the increased availability of oxygen, oxyacetylene torches replaced less effective air-acetylene torches used in welding. Although this advance was enjoyed only in Europe up to 1906, it spread along with the air liquefaction industry to other continents and is still in widespread use today.
The British Oxygen Company introduced the air liquefaction process to the U.S. at the world’s fair in St. Louis in 1904. By January of 1907, the Linde Air Products Company was established in Buffalo, New York. Within 14 years the worldwide production of liquid oxygen doubled. To reduce distribution costs, cryogenic vessels holding liquefied gases increasingly replaced gas cylinders.
However, inadequate insulation in the cryogenic vessels prohibited distribution over the large distances encountered in the U.S. Here the applications necessitated improvements to the liquid cryogen industry. In 1900, the best thermally insulating containers were those invented in 1892 by Sir James Dewar. These laboratory-based doublewalled glass vessels used a high degree of vacuum in the space between the walls to achieve their insulating properties, but were impractical for industrial purposes.
The ‘‘powder in vacuum’’ design, developed and commercialized by Leo Dana at the Linde Division of Union Carbide in the 1930s and 1940s, provided adequate insulation while reducing vacuum requirements and improving constructability. In this approach, the doublewall space of metal containers is filled with carefully selected powders. Containers utilizing this design today transport liquid oxygen, nitrogen, and argon worldwide for periods of several weeks without significant evaporation.
The 1940s brought the first commercial liquefaction of natural gas to improve the distribution of the popular fuel to homes and industries. Natural gas, used from the mid-1800s, is obtained from underground cavities and is primarily composed of methane. The 600-fold increase in the density of liquid natural gas (LNG) as compared to the gas at ambient conditions, motivated the development of the LNG production facilities.
The first commercial LNG plant was installed in Cleveland, Ohio in 1941 and liquefied 200 cubic meters per day. A storage tank failure in 1944 and subsequent fire caused serious damage and loss of lives, delaying growth of the industry for over ten years. Subsequent installations, with improved safety, were established first as a floating barge in 1956, and later as permanent land-sites worldwide. Today, the multibillion dollar LNG industry transports LNG via 41,000-liter-tank trucks and 125 000-cubic meter-capacity ships.
While the liquid air industry was beginning in the U.S., the scientific race to condense the remaining unliquefied gas approached its finish line. On 10 July 1908. Kamerlingh Onnes at the University of Leiden succeeded in liquefying helium at 4.2°Kelvin. Liquid helium remained a laboratory curiosity until the invention of a convenient helium liquefier by Sam Collins of the Massachusetts Institute of Technology (MIT) in 1947.
In 1952 the National Bureau of Standards in Boulder, Colorado was established to develop a large-scale hydrogen liquefier. By the 1960s liquid helium and hydrogen were being used extensively for emerging programs in space exploration and superconductivity. Union Carbide’s development of multilayer insulation (MLI) or superinsulation enabled the 100-fold improvement to the cryogenic storage vessels that allowed large-scale transport of liquid helium and liquid hydrogen.
Date added: 2023-10-03; views: 729;
