The Laws of Thermodynamics and Living Cells
Living cells have developed highly efficient mechanisms to utilize the energy obtained from chemical fuels and light to carry out numerous energy-requiring processes in order to maintain themselves in dynamic steady states. When a cell fails to obtain energy, it will die and decay toward equilibrium with its surroundings.
To understand how the energy is extracted, stored and channeled into useful work in living cells, we address cellular energy conversions in context of the law of thermodynamics and the quantitative relationships among free energy, enthalpy and entropy.
The laws of thermodynamics are general principles that provide the quantitative description of heat and energy changes and chemical equilibria. These laws apply to all chemical and physical processes, including biochemical reactions (van Holde etal., 1998; Edsall and Gutfreund, 1983; Alberty, 2003). Their importance resides in the fact that they determine the conditions in which a biochemical reaction can proceed.
In thermodynamics, the field of observation is divided into two conceptual regions: the system and the surroundings. The 'system' refers to everything within a defined region of space, including all the constituent reactants, products, solvent of the reaction, and the immediate atmosphere; while the system and its surroundings together constitute the universe.
When the system does not exchange either matter or energy with its surroundings, it is considered isolated. If the system exchanges only energy and not matter with its surroundings, it is defined as a closed system. An open system is one that exchanges both matter and energy with its surroundings.
The first law of thermodynamics describes the principle of the conservation of energy. In any physical or chemical change, the total energy, E, of a system and its surroundings is constant, although the form of the energy may vary. In other words, the first law states that E can be changed only by the flow of energy as heat or by work. Consequently, energy can neither be created nor destroyed, it can only be changed from one form to another as shown in the mathematical expression below:
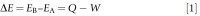
where EA and EB is the energy of a system at the beginning and the end of the transformation, respectively, Q is the heat absorbed, and W is the work done by the system. Note the change in energy of a system depends only on the initial and final states, independent of the transformation pathway.
When a given chemical reaction occurs under constant pressure, the amount of heat released or absorbed reflects the nature and number of chemical bonds altered during the course of the reaction. This heat of reaction is referred to as enthalpy, H, expressed as joules/mol or calories/mol (1 cal = 4.184 J).
Because the total enthalpy of a system cannot be measured directly, only the change of enthalpy, ΔH, is evaluated. If heat is being absorbed by the reaction, its ΔH is positive and the reaction is endothermic. On the other hand, if heat is generated by the reaction, the reaction is exothermic and its ΔH is negative. However, the first law of thermodynamics is insufficient to predict whether a reaction can occur spontaneously since some endothermic reactions do occur spontaneously.
Thus, a function other than DH is necessary to account for this observation. One such function is the entropy, S, expressed in unit of J/mol K. Note that entropy is a quantitative expression for the randomness or disorder in a system. When the products of a reaction are less complex and more disordered than the reactants, the reaction proceeds with a gain in entropy. It is worth mentioning that entropy is a central concept in biochemistry since life requires continual maintenance of order while increased randomness is the natural tendency.
The second law of thermodynamics states that a process can occur spontaneously, if and only if, the sum of the entropies of the system and its surroundings is >0. This indicates that the entropy of a system can decrease during a spontaneous reaction, if the entropy of the surroundings increases such that their sum is positive. However, the entropy changes of chemical reactions are not readily determined and the second law indicates that to determine whether the reaction can occur spontaneously requires one to know the value of the entropy changes for both the surroundings and the system of interest.
At constant temperature and pressure, a condition fulfilled by most biological systems, this constraint imposed by the second law can be obviated by using a different thermodynamic state function termed free energy (G) or Gibbs' free energy, derived from the combining of the first and second law of thermodynamics by Gibbs (1876-1878, 1878).
The basic equation is:

where ΔG is the change in Gibbs free energy of a reaction under constant pressure, P, and temperature, T, and ΔS and ΔH is the change in entropy and in enthalpy of the reaction, respectively.
Date added: 2024-06-13; views: 502;
