Generation of Cell Polarity
The previous section showed that polarized epithelial cells contain numerous membrane compartments (e.g., plasma membrane, Golgi apparatus, ER, endosomes, lysosomes), each with specialized functions bestowed by distinct biochemical compositions. All proteins originate in ribosomes and the ER, and are sorted to their appropriate destinations by vesicular transport pathways, guided by signals intrinsic to the targeted protein and extrinsic cues provided by cell-cell and cell-substratum contacts.
Pathways and Sorting Signals: All Roads Do Not Lead to Rome. Several pathways of flow are available to vesicles in polarized epithelial cells (Fig. 7). All proteins destined to be secreted or targeted to organelles are first imported into the ER, where they fold, oligomerize, and undergo partial N-linked glycosylation. They are then carried forward indiscriminately to the cis-Golgi network by coatomer-coated vesicles, a process that can be experimentally blocked by the drug brefeldin A, which disrupts the Golgi apparatus.
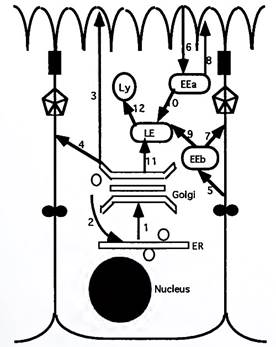
Figure 7. Principal pathways of vesicular transport. LE, late endosome; EEa, early apical endosome; EEb, early basal endosome; Ly, lysosome. 1, bulk transport from ER to Golgi; 2, return pathway for KDEL-containing ER resident proteins; 3, direct apical sorting (e.g., of GPI-linked proteins); 4, direct lateral sorting (e.g., for NPXY-containing proteins); 5, clathrin-mediated endocytosis from lateral domain; 6, clathrin-mediated endocytosis from apical domain; 7 and 8, recycling pathways; 9 and 10, transport from early to late endosome; 11, direct targeting from TGN to late endosomes; 12, targeting to lysosomes
Certain proteins contain an ER retention signal identified by the amino acids KDEL (Lys-Asp-Glu-Leu) at their COOH terminus; these proteins are selectively returned to the ER. This return pathway is microtubule dependent, and is blocked by nocodazole or taxol. All other proteins are transported through the Golgi stacks in the cis-to-medial-to-trans direction on coatomer-coated vesicles. Along the way, processing events such as N- and О-linked glycosylation are completed.
The trans-Golgi network is a major sorting station from which vesicles are targeted by a variety of pathways to the apical, basolateral, endosomal, or lysosomal membrane (Fig. 7). In the direct pathway, proteins are directly and vectorially delivered to the appropriate domain. In the indirect pathway, all newly synthesized proteins are delivered to the basolateral membrane; subsequently, those destined for the apical domain are removed by endocytosis and are transported to the apical membrane by trans- cytosis.
In the random pathway, the proteins are randomly delivered to both apical and basolateral domains. Proteins are subsequently sorted to the correct domain by selective endocytosis and transcytosis. All three pathways are used, and the predominant pathway employed depends on the cell type. For example, kidney tubule cells (both native and cultured) appear to employ predominantly the direct pathway to polarize basolateral proteins such as the Na,K-ATPase, whereas intestinal epithelial cells and hepatocytes have well-developed transcytotic pathways.
Until recently, it was erroneously believed that only the apical targeting of proteins is signal mediated and that the basolateral targeting of proteins represented a “default” pathway taken in the absence of an apical targeting signal. This assumption was based on the observation that it is usually the apical domain that is specialized for characteristic epithelial cell functions such as absorption and secretion. Many recent studies have challenged this view and have redefined basolateral sorting as a specific signal-mediated event.
Two classes of basolateral-sorting determinants can now be discerned. The first is related to clathrin-coated pit signals such as the NPXY motif (Asn-Pro-X-Tyr) in the cytoplasmic tails of proteins such as LDL receptor, vesicular stomatitis virus G protein, and Fc receptors. The second class of basolateral-targeting signals are found in proteins such as transferrin receptor and polymeric Ig receptor and contain a tyrosine residue that appears to be critical. Many proteins that are linked to GPI are found in the apical membrane, and splicing of a GPI anchor onto a normally basolateral protein makes it sort to the apical domain, suggesting that GPI anchors may serve as apical-targeting signals.
Proteins destined for the lumen of lysosomes, such as the acid hydrolases, are targeted from the TGN to late endosomes and lysosomes. These proteins contain a unique sorting signal in the form of mannose-6- phosphate (M6P) groups, which bind to M6P receptors at the TGN to form a receptor-dependent transport vesicle. When these vesicles reach late endosomes and lysosomes, the characteristic low intracellular pH in these organelles dissociates and releases the targeted proteins from the M6P receptor.
Endocytosis is a clathrin-mediated process by which localized areas of the plasma membrane invaginate and pinch off to form vesicles. Material endocytosed from either the apical or the basolateral domain first enters an early endosomal compartment that is domain specific. This allows endocytosed proteins to be recycled back to the original domain, unless they contain signals that target them to the other domain by transcytosis. Proteins that are not recycled are targeted to a common late endosomal compartment and then into lysosomes to be degraded.
Spatial Cues: The Guiding Lights. Epithelial cells arise at multiple times and places during morphogenesis from apolar progenitors. In the absence of extracellular contacts, single epithelial cells do not exhibit structural and functional characteristics of polarized cells. Cell polarity starts with a spatial cue, which is cell-cell or cell-substrate adhesion. Although some controversy exists on the precise hierarchy of signaling, it is clear that both pathways of spatial signaling are important.
Cell adhesion molecules, specifically E-cadherin, mediate cell-cell contact, and this alone may be sufficient to initiate a cascade of events that results in at least some forms of polarity. It is envisioned that these contact sites form “nucleating centers” for the recruitment of a spectrin-actin based cytoskeleton and associated signaling networks at the plasma membranes.
Other spatial cues emanate from integrin-mediated cell- substratum contacts very early in tissue morphogenesis. These interactions establish the apico/basal axis of polarity and also recruit an actin-based cytoskeletal complex and signaling molecules to the basal domain. Randomly oriented microtubules organize themselves with respect to the polarized cytoskeletal complexes, and the process of polarized protein sorting begins.
Bibliography:
1. Brown, D., and Stow, J. L. (1996). Protein trafficking and polarity in kidney epithelium: from cell biology to physiology. Physiol. Rev. 76, 245-297.
2. Clark, E. A., and Brugge, J. S. (1995). Integrins and signal transduction pathways: The road taken. Science 268, 233-239.
3. Cole, N. B., and Lippincott-Schwartz, J. (1995). Organization of organelles and membrane traffic by microtubules. Curr. Opin. Cell Biol. 7, 55-64.
4. Devarajan, P., and Morrow, J. S. (1996). The spectrin skeleton and organization of polarized epithelial cell membranes. Curr. Top. Membr. 43, 97-128.
5. Drubin, D. G., and Nelson, W. J. (1996). Origins of cell polarity. Cell 84, 335-344.
6. Gumbiner, B. M. (1996). Cell Adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 84, 345-357.
7. Matter, K., and Mellman, I. (1994). Mechanisms of cell polarity: Sorting and transport in epithelial cells. Curr. Opin. Cell Biol. 6, 545-554.
8. Rothman, J. E., and Wieland, F. T. (1996). Protein sorting by transport vesicles. Science 272, 227-234. 9. Schekman, R., and Orci, L. (1996). Coat proteins and vesicle budding. Science 271, 1526-1533.
Date added: 2024-07-02; views: 412;
