Restoring gradients: sodium ions
The continuous inward flow of sodium, which is indispensable to supply the cell with the glucose necessary for ATP production, slowly leads to a decrease in concentration differences between the outside and the inside of the membrane, with a progressive decrease of the electrical and chemical gradients. The consequence of this process is that the cell is no longer able to produce energy, loses its ability to do work and dies.
In order to overcome this impediment and maintain optimal function over time, the glucose transporter must be paralleled by a membrane protein that is able to maintain low sodium concentration inside the cell, moving sodium outwards against its gradient. This mechanism could be seen as basic maintenance of cellular homeostasis. In this case, the energy needed for the work is fully furnished by the cell in the form of ATP.
The model of the membrane protein, out of an infinite number of possible models, must have a) a high-energy ATP binding site for the molecule, b) high-affinity sites for the specific ion to be transported, and c) allosteric sites other than the specific sites and the ability to be modulated by conformational changes in parts of the protein itself.
Like sodium/glucose co-transport, the model is based on a repetitive cycle. In the resting state, the most likely conformation of the transporter must be that at the lowest level of potential energy (Figure 3.12; spring at rest). The specific high-affinity binding sites are facing into the cell (Figure 3.12 1).
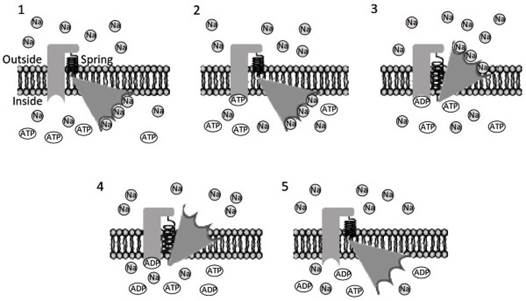
Figure 3.12. Hypothetical working model of the protein capable of transporting sodium ions outside the cell against the gradient. Sodium ions (Na) are transported by means of specific high affinity sites using the energy provided by an ATP molecule.
The cycle starts with the transporter in a position at low potential energy (1) (spring discharge) with the high affinity binding site facing the internal side of the membrane. Na ions binds the high affinity binding site, allowing ATP attachment (2).
Following saturation, the phosphorylation reaction ATP-ADP gives the energy to cause a conformational change in the transporter (3), taking the transporter to a high potential energy level (stressed spring). In this position, there is a drastic decrease of the binding strength for Na ions, which are released outside (4). The free binding sites and ADP detachment are the triggers for releasing the potential energy accumulated in the spring, with the transporter moving back to the starting configuration (5)
In this condition, the transporter has a high probability of being saturated despite the low concentration of sodium ions within the cell. Saturated sites can lead to the uncovering of an ATP binding site (Figure 3.12 2).
Phosphorylation of the transporter induces a conformational change, exposing the binding sites loaded with sodium ions externally (Figure 3.12, 3), in a position of high potential energy (stressed spring). As a consequence of the conformational changes, there is a drastic decrease in the sodium binding sites' affinity.
The sodium ions are released outside the cell, despite its high concentration (Figure 3.12, 4). Finally, ADP detaches from its specific site, allowing the transporter to change conformation back to its initial position, due to the potential energy accumulated in the stressed spring (Figure 3.12, 5).
Date added: 2024-07-02; views: 498;
