The transport of glucose
First of all, it must be assumed that the transporter is a transmembrane protein. There therefore must be a specific pore allowing sodium ions to pass through the membrane in order to exploit the potential energy, with a portion capable of binding glucose molecules.
If we consider proteins, it is well known that they can take on an infinite number of conformations completely at random as a result of temperature and thermal agitation, with frequencies as high as 1000 transitions per second.
It can be assumed that because of these conformational changes and in the absence of external conditioning, the transporter has a high probability of finding itself with the glucose binding sites (schematically three sites in Figure 3.11) facing inwards into the primordial cell, with the glucose binding site saturated and the sodium ion pore predominantly closed (1).
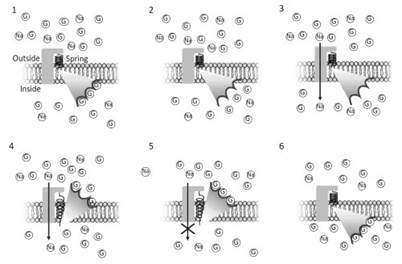
Figure 3.11. Functional model of the membrane element capable of transporting glucose (G) inside the cell. The cycle starts with the transporter at its lower level of potential energy, facing the internal side of the membrane and with glucose molecules saturating the binding sites (1).
In this condition, glucose is released due to the drop in the affinity of the binding sites (2). This causes the opening of the Na channel (3) that induces a conformational change in the transporter (4). In this position, at a high potential energy level (stressed spring), there is an increase of the binding site affinity for glucose. Once all the sites are occupied, the channel closes (5) and the system is free to revert to the original, low potential energy position (6)
Glucose is released because in this position, at a lower level of potential energy, glucose binding sites are at low affinity (2). Glucose release triggers the opening of the sodium channel (3). The inflow of sodium ions from outside gives the energy to cause a conformational change in the transporter. The spring in Figure 3.11 represents the intrinsic energy level of the transporter.
In (4) the transporter is at its maximum potential energy, with the binding sites facing the external side of the membrane. In this condition, the affinity for glucose increases. After the binding sites are saturated (4), the channel closes (5) and the transporter returns to its original position at a low level of potential energy (6).
This molecular model of glucose transport respects the physiological principle that biological systems are always in a state of dynamic equilibrium. The transporter is maintained at high potential energy by the continuous flow of sodium ions. Binding site saturation is the trigger to induce the ion channel to close and to allow the transporter to return to its original conformation at low potential energy.
The sequence of events just described is one of the possible models of a molecular mechanism designed to ensure a constant supply of fuel, glucose, which is continuously consumed by the mitochondria to produce energy in the form of ATP. A mechanism such as this is called co-transport.
Date added: 2024-07-02; views: 461;
