Restoring gradients: sodium ions and potassium ions
A cell that could select a membrane protein capable of coupling transport against the gradient of the sodium ion and the potassium ion would be evolutionarily advantaged, in that it could use only one ATP molecule for each transport cycle of both ions, increasing the differences in ion concentrations across the membrane and hence, ultimately, the chemical gradient for each ion.
Cell physiologists, many years ago, were able to identify and functionally characterize a ubiquitous membrane protein termed the Na/K pump. However, even for this membrane protein, the molecular mechanism is still obscure. Nevertheless, an effective functional model can be built that combines the transport mechanism of the sodium ion (Figure 3.12) with that of the potassium ion (Figure 3.13).
The transporter protein at rest must have the specific sites for potassium and sodium ions facing outwards and inwards from the cell membrane, respectively (Figure 3.14, 1). The sites also need to be high affinity, given the low relative concentration of ions in the environment towards which they are facing.
Saturation of the binding sites uncovers A TP binding sites (Figure 3.14, 2). Phosphorylation of the transporter protein promotes a conformational change able to charge both the saturated binding sites at a maximum level of potential energy (stressed spring), exposing bindingcomplexes externally for Na and internally for K (Figure 3.14, 3).
At the end of the conformational change, a drastic decrease in the affinity of the specific sites allows K and Na ions to be released despite the high concentrations in their respective environments (Figure 3. 14, 4). Finally, ADP detaches from its specific site, allowing the transporter to change conformation back to its initial position, due to the potential energy accumulated in the stressed spring (Figure 3.14, panel 5).
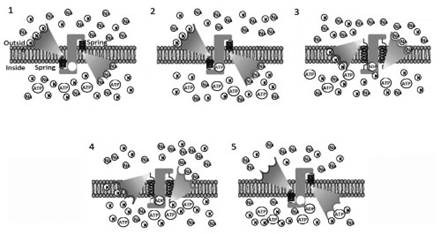
Figure 3.14. Hypothetical working model of the sodium/potassium pump. Sodium (Na) and potassium (K) ions are transported by means of specific high affinity sites using the energy provided by an ATP molecule. The cycle starts with the coupled transporter in a position at low potential energy (1) (spring discharge) with the high affinity binding sites facing the external side for K and the internal membrane face for Na. Na and K ions bind the high affinity site, allowing ATP attachment (2).
Following saturation, the phosphorylation reaction ATP-ADP provides the energy to cause a conformational change in the transporter (3), taking the transporter to a high potential energy level (stressed spring).
In this position, there is a drastic decrease of the binding strength for Na and K ions, which are released outside and inside respectively (4). The free binding sites and ADP detachment are the triggers to release the potential energy accumulated in the spring, with the transporter returning to the starting configuration (5)
This coupled transport is, from an electrical point of view, neutral. During its work, the distribution of charges across the membrane and the electrical potential remains unchanged. As we discussed previously (paragraph 3.1), the chemical gradient built by passive mechanisms for each permeable ion involved is responsible for a membrane potential difference in the order of -15, -20 mV.
The system is in a dynamic equilibrium, with potassium the only permeable ion. If sodium also becomes permeable, the potential difference will dissipate and sodium and potassium will soon be equal inside and out. Even if active transport is able to increase the chemical gradient of the two main ions (Figure 3.14), the membrane electrical potential will remain unchanged. To accumulate more potential energy across the membrane, it is necessary to select a membrane protein capable of asymmetrical ion transport.
Suppose that the cell has selected a protein capable of simultaneously transporting 3 sodium ions to the outside of the cell and 2 potassium ions to the inside (Figure 3.15). The transporter protein mechanism is the same as that already described (Figure 3.14). However, now for each cycle of the transporter, there is a negative charge left behind on the inner side of the membrane. Two of the three chloride ions in the internal environment compensate for the two potassium ions released by the transporter protein.
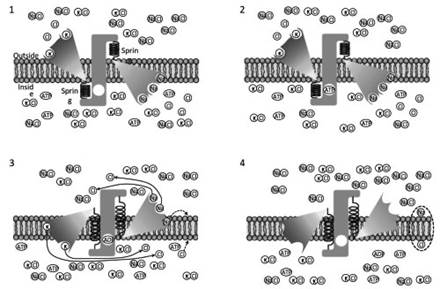
Figure 3.15. Associated transport model of sodium and potassium ions with an electrogenic function. Sodium (Na) and potassium (K) ions saturate their respective binding sites on the transporter (1), releasing the corresponding negative charges (Cl-). Once the transporter sites are saturated, ATP is allowed to bind to its specific site (2).
Hydroxylation promotes phosphorylation of the transporter molecule, which changes conformation (3). The excess of positive (external) and negative (internal) charges is compensated for by the membrane capacitor (dotted oval). Na/K ATPase electrogenic transport is one of the main contributors to increasing the potential difference across the membrane in most cells
Two chloride ions in the external environment compensate for two of the three excess sodium ions (continuous arrows in Figure 3.15B). The excess positive charge outside and the negative charge inside the cell create an unstable condition, as the principle of electro-neutrality of the solution is not respected.
The two charges attract each other close to the membrane (dotted arrows of Figure 3.15B) and stabilize by exploiting the capacitive properties of the plasma membrane and compensate for each other, even if they are physically separated (Figure 3.15C, dotted oval). The electro-neutrality of the solution is respected and the consequence is an increase in charge across the membrane.
The work performed by the asymmetrical active transport across the plasma membrane just described, which uses the chemical energy of ATP, is actually present in cells and is termed the sodium/potassium pump or more correctly Na+/K+ dependent ATPase or simply Na+/K+ ATPase (paragraph 4.3.2).
The number of ions transported is unknown and in the models of Figure 3.15, 3 binding sites for sodium and 2 for potassium have been used for simplicity. These numbers reflect the correct ratio Na/K of 3:2 operated by the transporter during repetitive cycling.
The transport performed by the Na+/K+ ATPase is electrogenic in that it produces, in addition to counter-gradient transport, an electrochemical gradient for sodium and potassium through a continuous accumulation of charges on the membrane capacitor and thus a large amount of potential energy in the form of electric potential difference. It is also one of the main mechanisms responsible for the negative voltage across the membrane.
The concentration of ATP inside the cell is constantly high due to continuous synthesis by the mitochondria. The Na+/K+ ATPase can therefore perform its electrogenic active transport action, which could result in an infinite increase in the negative charges inside the cell and a progressive and infinite hyperpolarization.
This does not occur because the active electrogenic transport is compensated for mainly by the background potassium permeability of the plasma membrane. In addition, Na+/K+ ATPase activity is voltage- and concentration-dependent. This results in a non-linear relation for the pump kinetics (Data Sheet 4.3).
Date added: 2024-07-02; views: 513;
