Characteristics of Feedback in the Hypothalamic-Pituitary-Adrenocortical Axis
The wide variation in total circulating corticosteroid levels, from the low nanomolar range during the daily circadian trough to the micromolar range during stress, provides a problem for feedback regulation by corticosteroids; the solution is to use two receptors of differing affinities to regulate the brain activation of the adrenocortical system.
In addition, there are three temporal domains for the inhibitory effects of corticosteroids and the brain also manages to modify the apparent efficacy of the negative feedback effects of corticosteroids under persistently stressful conditions, thus maintaining an HPA axis that is responsive under conditions in which circulating corticosteroid levels are higher than the usual set point.
Two Receptors Mediate the Feedback Effects of Glucocorticoids. Figure 1 indicates the problem that must be solved by a corticosteroid feedback system in order to control accurately the activity of the HPA axis under circadian and stressful conditions. During the trough of the basal rhythm, total cortisol levels range between 50 and 100 nM. Because at low values -99% of this represents cortisol bound to CBG, the free cortisol levels that can diffuse from the vascular compartment into the interstitial fluid to bathe cells range between ~0.5 and 1.0 nM.
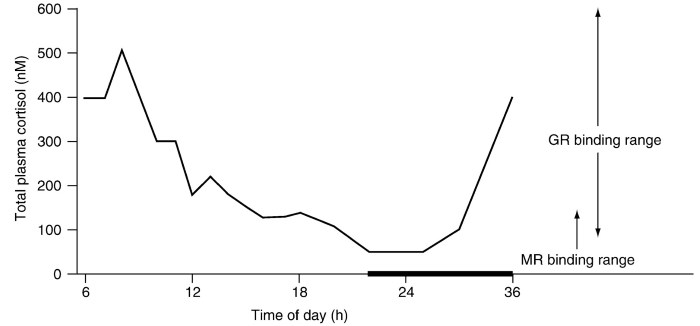
Figure 1. The two-receptor system that provides feedback information to the central HPA axis about adrenocortical performance. A hypothetical normal circadian rhythm in total plasma cortisol (CBG-bound and free) in humans. The dark bar on the abscissa represents the hours of darkness and sleep.
The peak occurs just prior to awakening and the beginning of the daily activity cycle. The trough (MR occupancy) occurs early in the hours of sleep and inactivity before values rise again. Stressor-induced increases in cortisol can reach micromolar concentrations. Clearly, if GRs are occupied, MRs are as well. The estimated occupancy of MRs and GRs by free steroid at the various levels of total steroid is shown on the right
By contrast, at the peak of the circadian rhythm and after stress, total cortisol ranges up to micromolar concentrations and free cortisol levels approach 100-300 nM or even higher. Because receptors characteristically bind hormone over a 100-fold range, it is clear that a single receptor for cortisol cannot control the range of cortisol concentrations required during the normal physiological excursions of the HPA axis. The solution is the use of two receptors of differing affinities to inform the brain of adrenocortical activity.
1. The MR is a high-affinity receptor (Kd 0.5-1 nM) that has roughly equally high affinity for aldosterone, cortisol, and corticosterone. In cells that are selectively responsive to aldosterone, such as the principal cells of the renal nephron, there is an enzyme (11-ß-hydroxysteroid dehydrogenase type II) that oxidizes the 11-hydroxyl groups of cortisol and corticosterone to the inactive 11-keto form.
Aldosterone exists as an 11-18-hemiketal in solution and is not oxidized by this enzyme; therefore, it can exert its actions selectively in these target cells. Cells that do not contain the enzyme but do have MRs bind all three steroids; however, cortisol and corticosterone circulate in higher concentrations and gain entry to the brain much more easily than aldosterone; therefore, these steroids primarily occupy MRs in the brain. In the brain, MRs have a highly discrete distribution, primarily in neurons in entorhinal cortex, limbic structures, and motor output neurons. There is a very low density of MRs in the neurons of the hypothalamus.
2. The GR is a lower-affinity (Kd 10-30 nM) corticosteroid receptor that prefers dexamethasone >> cortisol, corticosterone > aldosterone. The GRs are distributed in every cell type in the organism. The distribution of GR in the brain is very widespread in both glial cells and neurons. CRH neurons in hypothalamus are well endowed with GRs, as are the neurons in the limbic system, the monoaminergic systems, and others.
Both occupied MRs and GRs dimerize in the cell nucleus to alter transcriptional rates of tissue-specific genes. In addition, MRs and GRs in the same cell can heterodimerize and act transcriptionally. Moreover, it is increasingly clear that the hormone receptor complexes can function in protein-protein interactions with other transcription factors to alter transcription rates. At this time, the specific effect of occupancy of MRs and GRs on transcription is impossible to predict. Nonetheless, occupancy of MRs and GRs by cortisol or corticosterone serves to inhibit activity in the HPA axis, generally by an action on brain.
Feedback regulation in the animal and its utility. Using adrenalectomy and infusion of MR and GR agonists as well as treatment with specific antagonists in intact animals, it is clear that occupancy of the MRs is required to maintain ACTH secretion at low levels during the trough of the circadian rhythm. By contrast, at the circadian peak and during stress, the occupancy of both MR and GR restrains ACTH secretion, maintaining it within the normal limits observed in intact organisms. Because occupied GRs and MRs are powerful modulators of cell function, it is important to maintain their overall concentrations at tightly regulated levels.
Many corticosteroid-sensitive end points change their levels and activity when there is slight deviance on either side of the normal daily mean value. In studies of the daily mean of plasma cortisol in humans, values range from 6 to 8 μg dl-1 when levels collected over 24 h are averaged. Similarly, in rats, the 24-h mean corticosterone concentration ranges between 4.5 and 7 μg dl-1. Both species have relatively high circulating CBG levels. Above or below these ranges in unstressed humans and rats, untoward metabolic, behavioral, and immunological changes occur.
Date added: 2024-07-10; views: 507;
