Contaminated Sediments and Soils. Remediation Challenges
Owing to historically negligent waste handling and storage practices, contaminated soils and sediments are a persistent and toxic presence in subsurface environments and waterways. Contaminated soils and sediments act as source material for contaminant migration to surface water, ground water, and the atmosphere. Dispersed modern sources exist, such as urban storm runoff, combustion, and continued unintended release from waste storage and disposal, but their contribution pales in comparison to those associated with historic point discharges.
Examples include manufactured gas plants, chlorinated solvents from drycleaners, and mining sites. Hazardous waste poses threats to human and ecological health, and drinking water utilities. From 2004 to 2033, hazardous waste cleanup in the United States is expected to be $209 billion across 300000 sites. Several federal programs dedicated to cleaning up these sites include Superfund, the Resource Conservation and Recovery Act (RCRA), and the Department of Defense (DOD).
Contaminated Sediments. Sediments are a mixture of loose particles that accumulate in benthic zones of water bodies (e.g. rivers, wetlands, lakes, and harbors). They consist of eroded soils, detritus, and organic matter. Hazardous discharges include heterogeneous mixtures of heavy metals, metalloids, radionuclides, chlorinated organic compounds (e.g. polychlorinated biphenyls, PCBs), and polycyclic aromatic hydrocarbons (PAHs). Several pollutants have been banned including the renowned insecticide, dichlorodiphenyltrichloroethane (DDT), and PCBs. However, these and many other pollutants persist and do not degrade in aquatic environments for decades.
These legacy contaminants accumulate in fats of organisms, especially benthic organisms that inhabit sediment environments. This accumulation is known as bioaccumulation. Contaminants increase in concentration or biomagnify up the food chain, and humans risk exposure via fish or seafood consumption (see Further Reading section).
Contaminated Soils. Hazardous waste sites contain contaminated groundwater and/or soil. In most contaminated groundwater sites, nonaqueous phase liquids, or NAPLs, are also present. Figure 1 shows a continuous source of NAPL from a leaking storage tank. NAPLs migrate through the vadose zone, or unsaturated zone, and eventually reach the water table. Dense nonaqueous phase liquids (DNAPLs) have a specific gravity greater than 1 and sink to an impervious layer at the bottom of the water table.
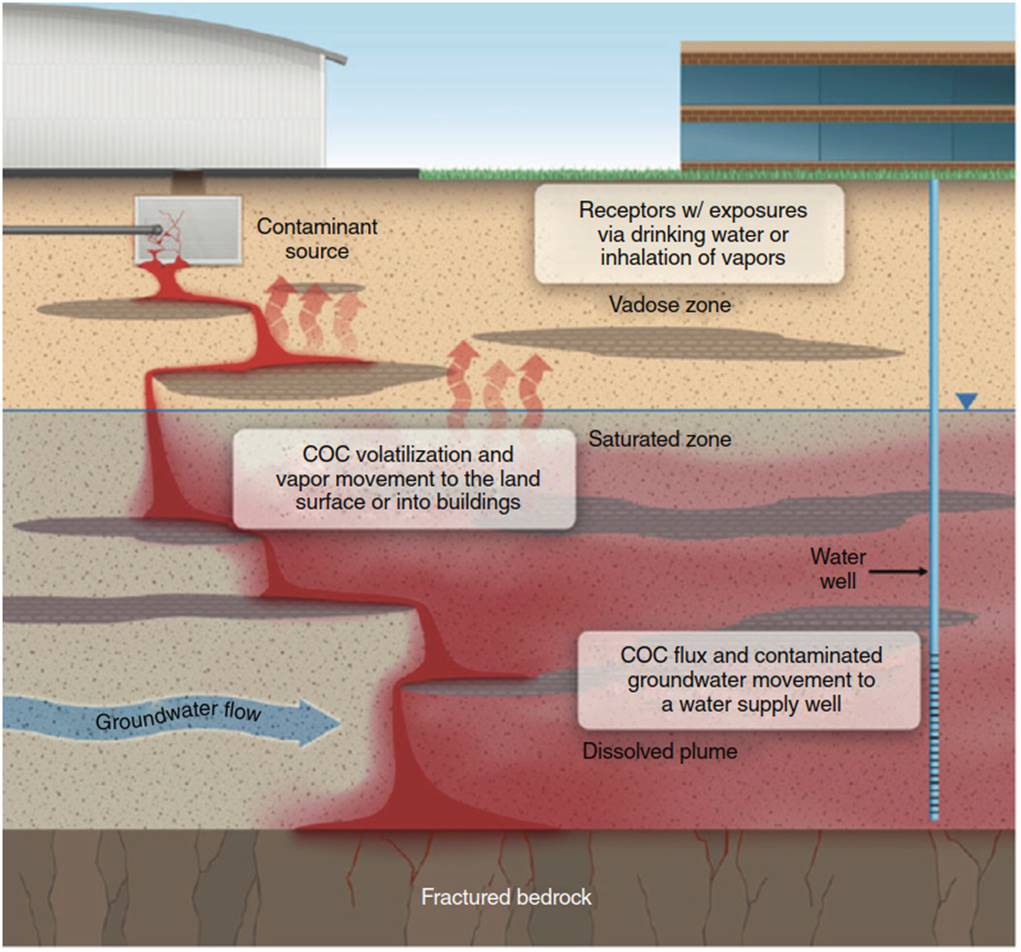
Figure 1. Subsurface profile of contaminant of concern (COC) release from an underground storage tank (UST). Source: In Situ Chemical Oxidation for Groundwater Remediation, Chapter 1 In Situ Chemical Oxidation: Technology Description and Status, Volume 3, 2011, Robert L. Siegrist et al. (eds.), © Springer Science + Business Media, LLC 2011, With permission of Springer
Trichloroethylene (TCE) is an example of a compound that forms DNAPL. Conversely, light nonaqueous phase liquids (LNAPLs) have a specific gravity less than 1. LNAPL floats on the top of the ground water table. NAPLs are difficult to locate and remediate, with DNAPLs being most challenging. A NAPL plume, although slowly moving, has the potential to reach drinking water wells and be an exposure concern to indoor environments by vapor intrusion.
Remediation Challenges. Contaminated sediments and soils have unique remediation challenges. Challenges specific to sediments include multiple contamination sources (point and nonpoint sources), complex exposure pathways to biota, and different flow conditions, depending on the water body (e.g. rivers, lakes, and harbors). Challenges specific to soils include physical media heterogeneities (e.g. bedrock, sand, and gravel), which affect contaminant hydrogeology and spatial variability of contaminants. This heterogeneity results in difficulties elucidating contaminant migration and targeting zones for treatment. Furthermore, over time soil contaminants migrate to inaccessible areas of the subsurface and act as long-term pollution sources.
Contaminated sediments and soils also share remediation challenges. Both sediments and soils are geosorbents for organic contaminants and metals. Contaminants interact with these geosorbents in a variety of ways, depending on the soil/sediment characteristics, contaminant type, and contact time.
Figure 2 illustrates several soil/sediment characteristics affecting mass transfer of contaminants. Soil organic matter (SOM) interacts with contaminants through sorption, or binding to soils/sediments. Dense and amorphous organic matter act as strong and weak sorption sites, respectively (see Further Reading section). Strongly sorbed contaminants are harder to remove from soils/sediments and are less available for release into the aqueous phase. Therefore, contaminants are less available for natural attenuation by microorganism-mediated biodegradation, or for targeted treatment.
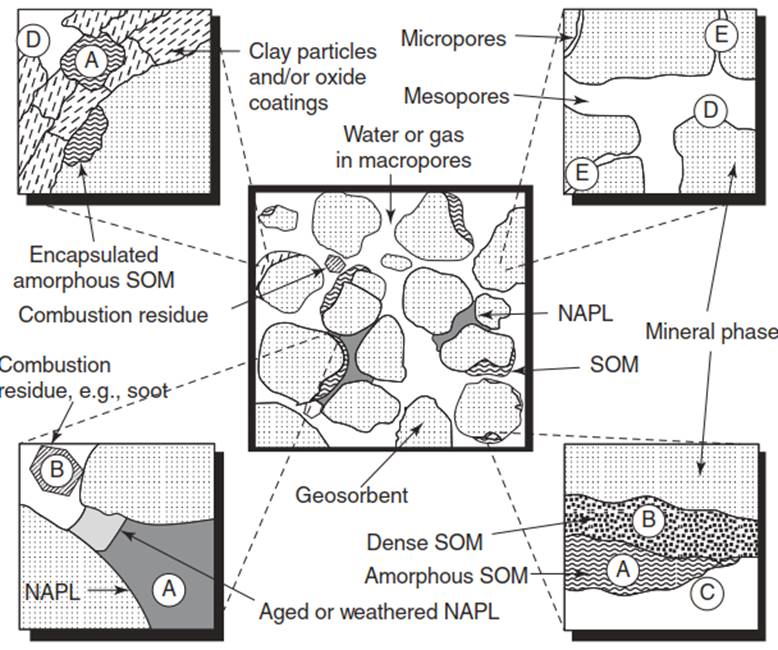
Figure 2. Soil/sediment heterogeneities include dense/amorphous soil organic matter, nonaqueous phase liquids, combustion residue, and varying pore sizes
Weakly sorbed contaminants are easier to remove, and are more available for treatment. Inorganic mineral phases (e.g. sand, silt, and clays) also pose remediation challenges. Mineral surfaces contain different pore sizes (macropores, mesopores, and micropores), which interact with contaminants via intraparticle diffusion. Contaminants trapped in micropores have the longest path length for diffusion and the slowest diffusion rate. Furthermore, contaminant-mineral interactions are influenced by mineral surface charge. Net mineral surface charge is determined by several factors including permanent structural charge, net proton charge as influenced by pH, and charge acquired from binding of ionic species Net surface charge strongly affects contaminant interactions with soils/sediments (see Further Reading section).
Contaminant type also contributes to remediation challenges associated with soil/sediment interactions. Three common contaminant types are neutral/nonionizable (without pKa) organics, ionizable organics, and metals. Neutral organic compound interactions with soils/sediments are affected by their degree of water repulsion, or hydrophobicity. Hydrophobicity is empirically expressed by the log of the octanol-water partition coefficient (logKOW). Neutral organic compounds with higher log KOW values are more strongly sorbed to soils and sediments than compounds with lower values.
LogKOW values have been shown to be linearly correlated to soil/sediment-water partitioning coefficients (Kd), as well as to soil organic carbon-water partition coefficients (KOC). In other words, compounds with higher KOW values increasingly partition to the organic carbon fraction of soils and sediments. Organic compounds containing functional groups such as phenols, carboxylic acids, and amines are ionizable and have pKa values. Solution pH controls protonation/deprotonation of these functional groups. Compounds have one more proton at pH values below the pKa, and one less proton at pH values above the pKa.
Depending on the functional group, compound pKa, and pH, the compound is ionized or neutral. Ionizable organic compound interactions with soils and sediments are primarily governed by electrostatic interactions with the mineral surface. The net mineral surface charge and pH-dependent compound speciation affect the Kd value of the contaminant. Metal interactions with soil and sediment surfaces, too, are affected by electrostatic interactions and pH conditions. As pH increases, metal ion adsorption to solid surfaces increases. At high pH values, electrostatic interactions between metals and deprotonated surface sites make it easier for metals to bind to soil/sediment surfaces. Metals can also bind to organic matter fractions of soils and sediments, affecting metal speciation and availability.
Contact time, too, affects contaminant-geosorbent interactions, and complicates remediation. Compound desorption rates from soils and sediments decrease with increased contact or residence time. This phenomenon is also known as aging. Therefore, aged, contaminated soils and sediments are less available for biodegradation and treatment by extraction.
Date added: 2025-01-04; views: 351;
