Fundamentals of Ultrasound. Acoustic Cavitation. Chemical Effects of Ultrasound
Ultrasound is an alternative sediment and soil remediation technology. Sonication offers an array of chemical and physical effects across a range of environmental systems. It can be coupled with other remediation technologies for synergistic contaminant treatment, and has the potential to be an in situ technology that actively reduces contaminant masses.
Acoustic Cavitation. Ultrasonic waves are longitudinal waves at a frequency above the audible range of humans, typically defined as greater than 16 kHz, which produce oscillating regions of high and low pressure. In liquid media, cavitation bubbles are formed when the pressure amplitude exceeds the tensile strength of the liquid during rarefaction or expansion of sounds waves. Bubble formation is facilitated by dissolved gas nucleation sites or “weak spots". An acoustic bubble expands during the rarefaction cycle, and in many cases immediately undergoes a violent, adiabatic collapse in the following compression cycle. The adiabatic compression of the gases in the bubble causes a high-energy release, resulting in ultrasound’s unique chemical and physical effects.
Chemical Effects of Ultrasound. Cavitational bubble collapse of concentrated sound energy yields immense temperatures and pressures. These cavitational bubble collapses produce localized “hot spots" in irradiated solution reaching average bubble temperatures of 4200 K, peak core temperatures of 20 000 K, and pressures of 500 atm, while lasting only nanoseconds. Figure 3 depicts three regions within this “hot spot," all exhibiting different chemical effects.
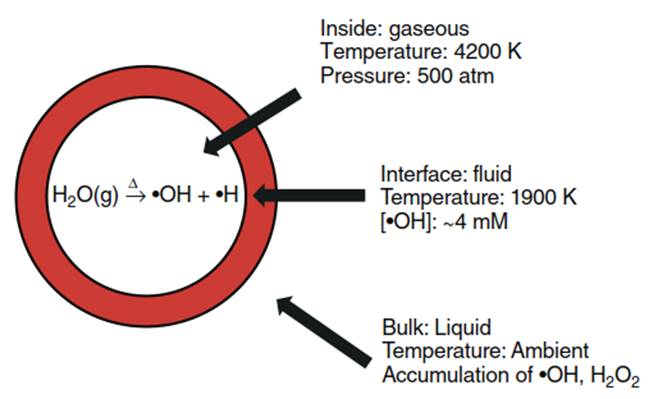
Figure 3. Three regions of acoustic cavitation
The interior, gaseous region of the cavitation bubble contains water vapor, gas, and volatile compounds. Extreme temperature and pressure result in thermal bond dissociation of water into •OH and •H. Volatile organic compounds that have migrated into the gaseous region degrade via thermolysis.
The second region is the bubble-water interfacial region characterized by large temperature and pressure gradients. High, local •OH concentrations (4mM) at the interfacial region are attributed to •OH migrating from the gaseous core to the bulk liquid. Hydrophobic, nonvolatile compounds that have partitioned to this region exhibit surface excess and degrade via •OH oxidation and thermolysis. The interfacial region may contain supercritical water. Though just a theory, the presence of supercritical water may enhance compound solubility and promote hydrophobic compound accumulation at the interfacial region.
The final region of the localized hot spot is the bulk liquid phase. Only 10% of •OH generated in the gaseous region migrates to the bulk phase because of solute interactions in the interfacial region. Hydroxyl radicals in the bulk phase combine with •OH to form hydrogen peroxide, H2O2, or react with the solute or solvent. Despite the extreme conditions of the gaseous and interfacial regions, bulk phase temperature and pressure remain at ambient conditions.
The unique chemical and thermal effects in each region of collapsing cavitation bubbles have been employed to degrade a variety of organic contaminants in aqueous media. Some examples include PCBs, PAHs, chlorinated solvents, and pharmaceuticals and personal care products (PPCPs). Ultrasound, along with other AOPs, therefore can be used for hazardous waste remediation.
Free-radical reactions can be studied using a spin trapping technique called electron paramagnetic resonance, or EPR, spectroscopy. Spin trap reagents trap short-lived radical species, forming stable spin adducts. Spin adducts allow for quantification of radical yields and radical intermediate concentrations, improving the mechanistic understanding of acoustic cavitation and sonochemical reactions. A spin trapping study conducted by Makino et al. presented evidence for •OH and •H formation in aqueous solutions in the interior, gaseous region of cavitation bubbles using different spin traps. Spin traps and radical scavengers (e.g. methanol and ethanol) were simultaneously used to compete for radical species. High radical scavenging by O2 indicated that radical species primarily form in the gaseous region of cavitation bubbles.
Additionally, spin trapping studies have analyzed radical speciation during contaminant degradation via ultrasound. Sonolysis of organic liquids produces multiple organic radical species (e.g. •R and •R') by thermolysis or H-abstraction. Spin trapping studies on ultrasonic degradation of aqueous contaminants have corroborated nonvolatile solute degradation at the interfacial region and volatile solute degradation in the gaseous region.
Date added: 2025-01-04; views: 378;
