Trans-Generational Stress Memory
Perception of a potential stress factor clearly influences the physiology and development of a plant in various ways. This is apparent from several phenomena discussed in previous paragraphs (Sects. 2.2-2.4). Exposure to cold induces a hardening or acclimation process that results in improved cold tolerance. Long-term cold experience inactivates repression of flowering (vernalisation). Attack by a pathogen or herbivore triggers systemic changes that result in a state of greater resistance (systemic acquired resistance). A fourth type of phenomenon is summarised as “priming”.
The response of a plant individual to a stress factor is shaped by a previous exposure in such a way that the plant is better able to cope with the stress factor because of the conditions it has experienced before. An example is the positive effect that colonisation by beneficial rhizobacteria can have on the pathogen resistance of a plant. This is also referred to as induced systemic resistance. All of these phenomena constitute a kind of memory. The plant’s ability to cope with a stress is improved through the changes elicited by preceding conditions (Hilker et al. 2016).
A fundamental question of plant biology and also of molecular stress physiology is whether the experience of stress is passed on to the offspring as well. If this were the case, it is easy to envision how such trans-generational stress memory would improve the ability to thrive in a habitat characterised by certain stress factors. Indeed, it has been observed repeatedly that the offspring of parental plants exposed to a stress show greater stress tolerance than the offspring of genetically identical parental plants not exposed to the stress. This could be interpreted as evidence of a transfer of experience to the filial generation, which is reminiscent of the concept proposed by Jean-Baptiste Lamarck: an individual acquires an adaptive trait and this trait is then inherited by its progeny. The basis for such a transfer would be epigenetics. This term refers to heritable changes in gene expression without changes in the DNA sequence.
The mechanisms underlying epigenetic phenomena are:
- Covalent modification of DNA by methylation of mostly cytosine; such methylation especially of promoter regions can silence genes
- Covalent modifications of histones (the proteins DNA is wrapped around in nucleosomes); specific modifications (e.g. acetylation, methylation, phosphorylation) can result either in chromatin that is transcriptionally active (classically called euchromatin) or in chromatin that is transcriptionally inactive (heterochromatin); the specific modifications and their consequences for the chromatin state are universal and are referred to as the histone code
The positions of these modifications, also called epigenetic marks, are often influenced by non-coding RNAs in a sequence-specific manner. For example, small RNAs guide DNA methylation (for more details, see molecular biology textbooks).
Gradual inactivation of FLC expression during vernalisation (Sect. 4.2.2) represents a form of memory conferred by epigenetic marks—in this case, histone modifications. As discussed, the memory is passed on to daughter cells in the meristems and therefore can be stable for several months. The transfer is mediated by an enzyme machinery that recognises histone modifications and introduces corresponding changes during cell division. We have also seen that the memory is erased when gametes are formed, so the memory of winter is not inherited by the offspring.
This is essential for the vernalisation to serve its biological function. The key question regarding trans-generational stress memory is whether, in contrast to vernalisation, a change in chromatin that is caused by exposure to stress is passed on to the filial generation—in other words, whether stress-induced epigenetic changes can be meiotically stable. Current knowledge suggests that mitotic stability is widespread (as is the case for FLC), but there is very little direct evidence to support meiotic stability (Iwasaki and Paszkowski 2014) (Fig. 2.38).
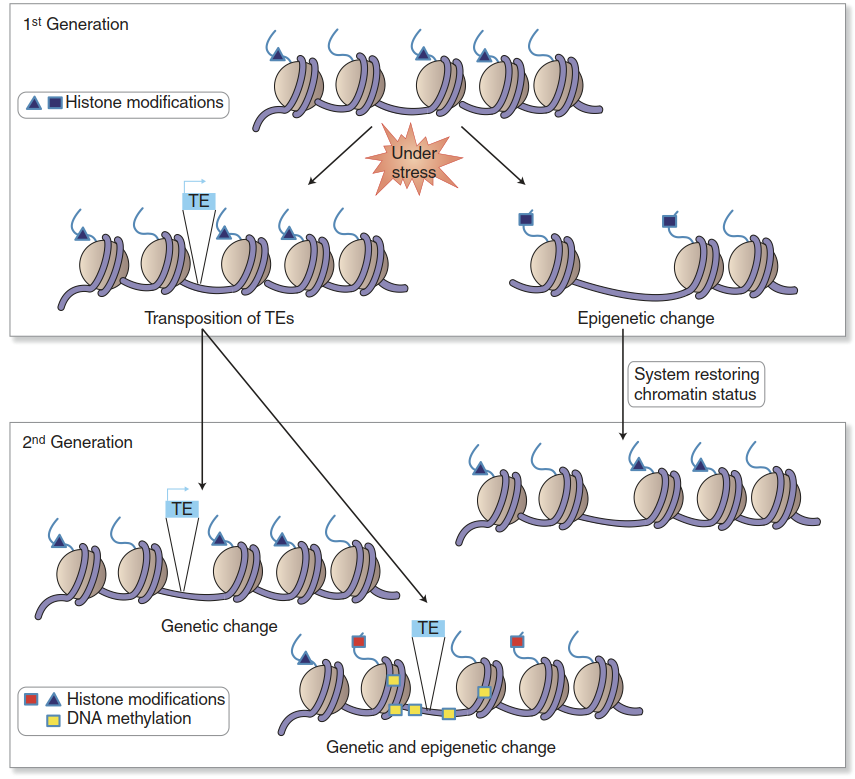
Fig. 2.38. Epigenetic changes under stress. Stress exposure causes epigenetic changes, such as histone modifications, that influence the chromatin state and thereby transcriptional activity. Such changes are mostly transient because they are reset during meiosis and therefore are not passed on to the next generation. Positive stress memory effects could be due to the activation of transposons (TE: transposable elements)—mobile DNA that could, upon transposition, influence the chromatin stage of neighbouring genes and affect transcriptional activity (Iwasaki and Paszkowski 2014)
Thus, the existence of true trans-generational stress memory is insufficiently supported to date. Several criteria have to be met before one can truly diagnose trans-generational memory (Pecinka and Mittelsten Scheid 2012). Among them are the following: the stress memory effects should be documented for more than two non-stressed generations to rule out that in fact non-epigenetic changes caused by stress in the egg cell are carried over into the next generation (so-called maternal effects); the stress treatment of the parents should occur early in life and well before gamete formation in order to minimise such carry-over; and better stress acclimation of the progeny should be demonstrated under natural conditions. This is important also because one could just as well postulate that stress memory effects could be disadvantageous for the filial generation by restraining their ability to acclimate. Thus, resetting of epigenetic changes during meiosis, as seen in FLC, may in fact confer better adaptation than stress memory (Iwasaki and Paszkowski 2014).
What could be alternative explanations for the observed stress memory effects, that is, the greater stress tolerance of individuals originating from parents that were exposed to the stress? The most obvious ones are the aforementioned maternal effects. Acclimative changes occurring during reproduction, especially in the egg cell, influence the individual developing from the fertilised egg. A second explanation could be the well-documented activation of mobile genetic elements— transposons—during stress (Fig. 2.38), which was postulated by Barbara McClintock, the discoverer of transposons. Transposon mobility can influence the chromatin state in neighbouring regions of the DNA and thereby influence gene activity. Such changes could improve the stress tolerance of the progeny but would not represent stably inherited epigenetic marks.
References: Achard P, Cheng H, De Grauwe L et al (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311:91-94
Alonso JM, Stepanova AN, Leisse TJ et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653-657
Amasino R (2010) Seasonal and developmental timing of flowering. Plant J 61:1001-1013
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373-399
Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796-815 Assmann SM (2013) Natural variation in abiotic stress and climate change responses in Arabidopsis: implications for twenty-first-century agriculture. Int J Plant Sci 174:3-26
Bewley JD (1997) Seed germination and dormancy. Plant Celt 9:1055-1066
Bohlenius H, Huang T, Charbonnel-Campaa L et al (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312:1040-1043
Boyer JS (1982) Plant productivity and environment. Science 218:443-448
Date added: 2025-01-13; views: 408;
