Mean Residence Times. Loss of Resources
In a well-balanced system, formation of biomass and mineralisation of organic matter would occur simultaneously and be in equilibrium. However, this equilibrium does not occur in the real world, as there are considerable time lags between use (for biomass formation) and release (via mineralisation) of resources, described by the mean residence time (MRT; Eq. 13.5) in the respective compartment.
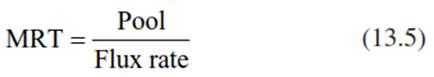
where Pool is given in mass per area and Flux rate is given in mass per area and time. Thus, the unit of MRT is time.
For example, leaves are synthesised within one year, but the foliage stays on a plant maybe for years and the litter is decomposed over many months to years—that is, nutrients bound in leaves will, on average, become available for further uptake and growth only after 2-8 years (Persson et al. 2000). The mean residence time of these nutrients in the foliage is thus 2-8 years. In wood, nutrients may be bound for more than 100 years. The decomposition of a tree trunk takes decades, and thus the delay between uptake of resources and return of the same resources is a lot longer for wood than for leaf litter.
The accumulation of litter is thus a sign that the shedding of leaves or needles exceeds the capacity of soil organisms to decompose this litter. Fast decomposition is limited either by lack of the organisms capable of mineralisation this litter, by plant compounds that are difficult to metabolise, by unfavourable climatic conditions, by stabilisation in the soil, or by a combination thereof. An open stand typically provides better conditions for mineralisation (higher soil temperature and moisture, affecting soil organisms) than a closed stand. Evergreen needles are less decomposable than deciduous leaves. Woody material decomposes more slowly than foliage. Depending on the decomposability of organic matter, different fractions of soil organic matter have very different ages, ranging from very young (recent soil carbon, up to 30 years old) to soil organic matter, which might be older than 1000 years.
Loss of Resources. Terrestrial ecosystems are thermodynamically open systems—that is, energy and matter get lost. Some of these losses are unavoidable, since they occur naturally either as part of the energy budget, during background soil biogeochemical and plant ecophysiological or defence processes, while some of these losses have natural agents such as wind, water and fire. But also anthropogenic activities play a major role—for example, human-induced fires, forest and agricultural management, and environmental pollution.
The resources that are lost include:
- Water: owing to the energy budget, run-off and infiltration into deeper soil horizons
- Mineral particulate matter: during dust storms and owing to water erosion
- Organic particulate matter: owing to erosion and during fires
- Carbon and nutrients (natural): via decomposition and nitrification, respiration and volatile organic carbon (VOC) production, infiltration into deeper soil horizons, run-off, erosion after natural disturbances
- Carbon and nutrients (anthropogenic): during fires and owing to management of terrestrial ecosystems
- Increased nutrient losses: owing to soil acidification after environmental pollution (N deposition)
Some of these resources lost from one ecosystem can be beneficial to other ecosystems. For example, mineral dust transported across the Atlantic Ocean fertilises the Amazonian forest with basic cations (Bristow et al. 2010). On the one hand, some losses are core for closing biogeochemical cycles. Denitrification (the release of N2) is the only natural process capable of closing the global N cycle. On the other hand, some losses from terrestrial ecosystems have detrimental effects on ecosystem health—of the ecosystem losing the resource and of the ecosystem(s) receiving it. For example, accelerated nitrate and cation losses occurred because of soil acidification after environmental pollution (N deposition) and resulted in decreased forest health and tree growth across Europe in the 1980s and 1990s.
Chemical conditions in the soil are primarily dependent on the constitution of the original bedrock. However, these conditions are changed as a consequence of the mobilisation and uptake of nutrients by microorganisms and plants—for example, when seasonality of plant growth, and thus demand, is decoupled from supply via microbial decomposition and nitrification processes. Since nitrate in the soil is not bound to minerals or organic matter, it can be leached into deeper soil horizons and/or the groundwater, taking along cations. For example, if nitrate is formed in autumn, when most plants stop growing, large nitrate and cation losses occur during winter. In summer, however, the nitrogen requirement of the vegetation can even exceed the supply from the soil. This asynchronous pattern of supply and demand leads, in the end, to changes in habitat conditions, with local overexploitation or accumulation of intermediary products, given that degradation is impeded by decreasing pH (e.g. raw humus).
But also anthropogenic activities, such as acid deposition with strong acids (H2SO4, HNO3), affect chemical conditions in the soil. The rate of soil acidification depends on the mineral constitution of the bedrock and the cumulative acid inputs. On limestone soils with a high CaCO3 content, incoming acid deposition is at first balanced by weathering of carbonate (Eqs. 13.6 and 13.7; Fig. 13.11a):
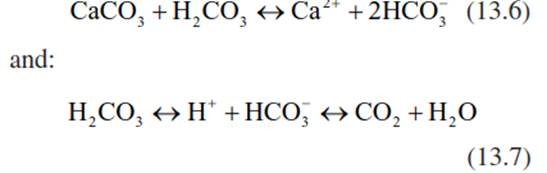
The Ca2+ ions that are released occupy the charges that are freed at the soil exchange sites. With time, CaCO3 will be continuously consumed, the soil pH will further decrease and a reversible exchange of cations will occur with clay minerals and organic matter. Under continuing acid inputs and thus loss of cations from the exchange buffer, the H+ buffering will be achieved by metal oxides and hydroxides, leading to a pH-dependent increase in the availability of certain metal ions. For example, Mn2+ becomes mobile at a pH between 5 and 4.2. At a pH of 4.2, the soil reaches another stable buffer system, the one buffered by Al hydroxides. The iron buffer range (with Fe3+) is reached below pH 3.8. The availability of ions is very variable during the course of this process, and each element is specifically dependent on the pH of the soil solution (Fig. 13.11b).
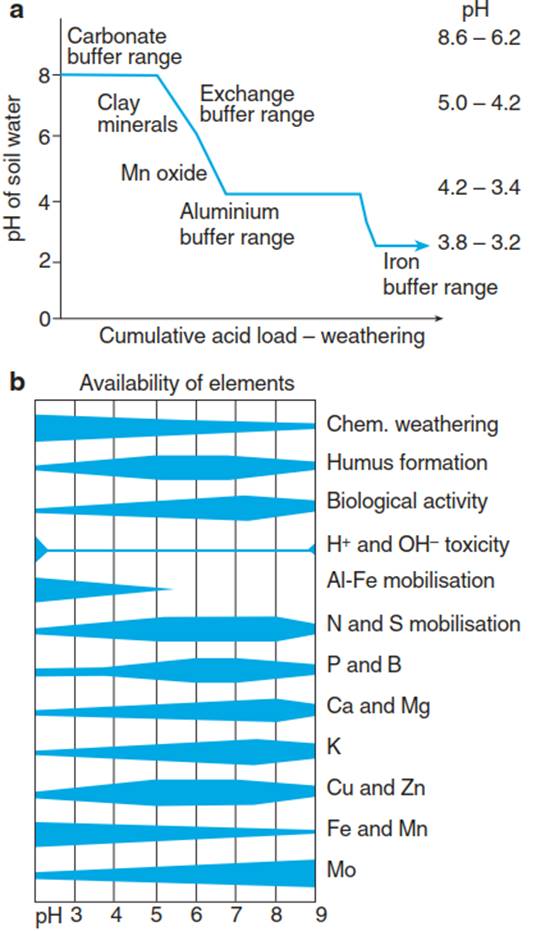
Fig. 13.11. Change of nutrient availability with soil pH. a Changes in the pH of the soil solution with continuing weathering as a consequence of the cumulative proton stress—that is, acid deposition (after Schulze and Ulrich (1991)). b Availability of nutrients depends on the pH of the soil solution (Larcher 2003)
The chemical changes in the soil are reversible—that is, by fertilisation or liming, provided that the clay minerals are not restructured. As soon as the crystalline structure of silicates and clay minerals is changed (e.g. by dissolving the Al lattice in a replacement of alkaline cations with protons; Chap. 11), a reversal into the original state is no longer possible, not even by abundant supply of cations. The ecosystem’s health is damaged irreversibly.
Date added: 2025-02-05; views: 344;
