Impacts on Species Composition
Disturbances not only change the element or substance pools and associated fluxes, but also affect the composition of species within the ecosystem. Areas suitable for growth are opened that were previously occupied by other species now weakened or killed by the disturbance. The remaining, surviving organisms may spread or new species may appear because seedlings can establish without competitors, for example, after a stand-replacing fire. The greatest number of species is found with average disturbance intensities (the intermediate disturbance hypothesis; Fig. 13.8) (Connell 1978) (Chap. 20, Sect. 20.2). If the frequency of disturbance increases, only a few specialists remain.
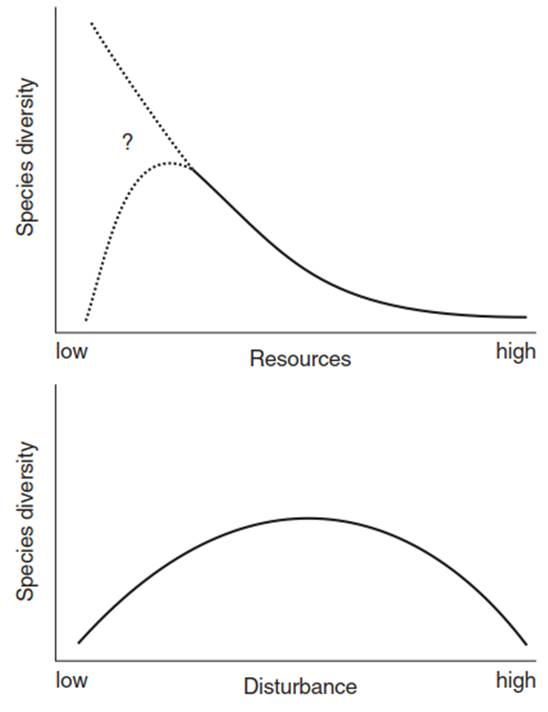
Fig. 13.8. Conceptual model of the relationship between species diversity and resource availability, and between species diversity and habitat disturbance. Species diversity reaches a maximum with average ecosystem disturbance. (After Hobbie et al. (1994))
At sites where fires occur regularly, vegetation has adapted to fires, with thick bark (e.g. Sequoia in North America), capacity to resprout (e.g. many Mediterranean shrubs), the presence of lignotubers (i.e. thick swelling of the root crown, filled with starch, lots of meristems to resprout, e.g. Eucalyptus in Australia), or cones that need high temperatures to open and release seeds (e.g. Banksia in Australia). Sometimes, only the outer layer of the woody stems is burned and turns to charcoal, so regeneration from living tissues at the base of the stem is still possible.
The Australian grass tree (Xanthorrhoea) even contains a resin that is difficult to burn and protects the stem from fire. In savanna systems, the sparse trees (between 10% and 50% cover) can typically survive (Fig. 13.9a, b); the grasses present in these systems can regrow from meristems close to the ground or below the ground, and herbaceous vegetation can germinate from the seeds below the ground (seed bank) and establish very quickly. Lush growth of herbaceous species is thus often a consequence of surface fires, as many nutrients become readily available in the ash (Fig. 13.9c) and shortly afterward because of high mineralisation rates in dark soils (the albedo effect), despite large fractions of C and N being lost from the system. Some plants— the so-called pyrophytes—even need fire to stimulate flowering or open their fruits (Fig. 13.9d).

Fig. 13.9. Examples of vegetation adapted to fire. a, b Fire vegetation on Mount Kilimanjaro (Shira Plateau). a Erica arborea as stunted vegetation that regenerates from charred branches. On average, there is a fire every 3-5 years. b Charred E. arborea at a site with less frequent fires. The bifurcated twigs show that the plant had survived at least one fire. Between the last and the most recent fire a substantial trunk had developed, and the intensity of the fire was therefore high. Both pictures were taken 2 years after the fire. Rejuvenation of the bushes had occurred, with bushy growth on the frequently burned site, where little biomass had accumulated between fires. In contrast, growth from the charred thick trunk was comparatively sparse. In the foreground is Helichrysum splen- didum, a typical fire indicator. c Vigorous germination of Festuca obturbans and Kniphofia thomsonii after an intense fire in the alpine zone of Mount Kilimanjaro. In the foreground are charred twigs of Erica arborea and Erica trimera. d Fruits of the Australian Hakea (Proteaceae) open only after a fire
Often, continuous slow forcing and sudden disturbances create new growth conditions that favour different plant species from those prior to these events. But the new species might also feed back on other organisms and organism groups in the ecosystem (Chap. 19, positive and negative feedbacks), as well as on the environmental growth conditions. Thus, they create an environment that favours their own species, at the expense of its native competitor. Some of the underlying mechanisms at the molecular and plant levels have been already discussed in Parts I and II (e.g. Chap. 12), but new (feedback) processes come into play when we are considering the ecosystem level (Sect. 13.3, emergent properties; Chap. 19, positive and negative feedbacks). An example from North America illustrates this situation very nicely:
The Mediterranean grasses Bromus tectorum and Bromus rubens successfully invaded the North American Artemisia tridentata shrubland since the end of the nineteenth century, which led to a marked reduction of Artemisia (West and Young 2000) and a pronounced change in the fire frequency. Bromus is a winter annual, which germinates in the autumn, establishes and grows over winter and completes its life cycle in late spring/ summer, avoiding most of the hot and dry summers as seed. Artemisia is a long-lived shrub, which grows throughout the summer and flowers in late summer/early autumn. It contains terpenoid compounds against herbivory and can resprout after a fire.
When Bromus was introduced accidentally to this perennial ecosystem, a plant with not only a very different life cycle but also very different ecophysiology was introduced (Germino et al. 2016). Bromus has very high water use early in the growing season before seed set, when water availability in the soil is still high. However, this means that the soil water pool is depleted faster and Artemisia, which must survive the dry summer period, does not have enough water later in the season. Thus, Artemisia dies, often without having formed seeds. In addition, the effect of differential water use is enhanced by fire. Because of the high fuel load with dead Bromus biomass during summer, the fire frequency has increased from 60-110 years to only 3-4 years (D’Antonio and Vitousek 1992).
This again favours Bromus, which germinates from seeds in the autumn, when its own standing biomass has been burned and its competitor, Artemisia, has been weakened or killed. Moreover, Artemisia cannot regenerate as fast as Bromus, and Artemisia seedlings are not able to compete with Bromus when competing directly. This example demonstrates that the consequences of resource use are quite different at the plant level versus the community level—that is, when competition for the same resource sets in and “saving” water might just provide water for a competitor.

Fig. 13.10. Invasive plant species. a Invasive species often spread along roads. Salsola kali (Chenopodiaceae) can be found close to roads in the semi-deserts of Utah in western North America. Salsola is a salt-tolerant species from Asian deserts. Away from the kerb of the road, the Mediterranean Bromus dominates. b Mediterranean Bromus in the Artemisia shrubland of Wyoming. Artemisia cover is already broken
Therefore, many successful invasive species have relatively high resource demands, not being very resource efficient (Fig. 13.10). This example also shows how changes in the native species composition owing to slow changes and disturbances can in turn affect the ecosystem’s environment. Fire frequencies have changed because Bromus produces a lot of standing biomass that burns easily and, as a grass, it can withstand fires easily and either germinate from the seed bank or regrow from root collar meristems. Thus, shortened fire intervals have favoured the invasive grass over the native perennial shrub.
Date added: 2025-02-05; views: 354;
