Creating different environments in ion composition
Potassium chloride and sodium chloride, derived from the melting of rocks that were very rich in them, were almost certainly the prevailing salts in water when it accumulated in a liquid state on the earth's surface, a process that occurred 3.9 billion years ago due to the cooling of the earth.
In that liquid environment, the first living organisms are thought to have formed between about 3.9 and 3.5 billion years ago, as these are the ages attributed to some microbial structures at a Greenland site (Nutman A., et al, 2016) and fossil cyanobacteria at a site in Western Australia (Campbell NA and Reece JB, 2004), respectively.
In the salt, each positive ion is surrounded by a number of negative ions, each of which is in turn surrounded by a number of positive ions, forming a compact and highly ordered crystal structure (Figure 3.3 A).
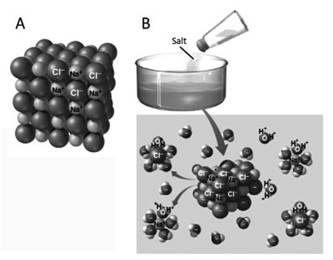
Figure 3.3. If a salt, which has an ordered crystalline structure (A), is placed in an aqueous solution (B), the positively charged cations separate from the negatively charged anions because they are surrounded by the dipoles of the water molecules
From a biophysical point of view, water molecules are dipoles with a negative charge fraction due to the oxygen atom and two positive charge fractions due to the two hydrogen atoms. The salt dissolves when the water molecules, moving freely by thermal agitation in a solution with a high concentration of water, have a high probability of inserting themselves in an oriented manner between the negative ions and the positive ions of the salt (Figure 3.3 B). The hydrated cations and anions diffuse in all directions due to thermal agitation and are distributed randomly and homogeneously throughout the solution (section 4.1.1).
This is done by respecting the principle of electro-neutrality of a solution, according to which every positive charge must correspond to a negative charge, and vice versa. Each ion has a characteristic hydration number, which indicates how many water molecules surround it, and this modifies, and in part determines, biophysical characteristics such as the mobility of the ion in solution and the permeability through the aqueous pores of a plasma membrane.
Consider a saline solution containing the same concentration of sodium chloride and potassium chloride: the sodium, potassium and chloride ions are distributed randomly and homogeneously throughout the solution (1 in Figure 3.4). If a nucleus with predominantly negative fixed charges is introduced into this system, for example a protein aggregate (2 Figure 3.4), the degrees of freedom of the system decrease because the fixed charges of the negative protein aggregate tend to restrain the sodium and potassium cations, which will form a layer around it.
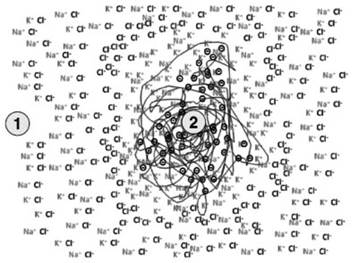
Figure 3.4. In a salt solution, the ions are homogeneously distributed (1), but the introduction of a negative protein nucleus (2) induces the formation of alternating layers of cations and anions
From a random and homogeneous distribution, we are now in a system able to separate charges. The excess of positive charges will now be compensated for by another layer of negative charges, consisting of chloride ions, which in turn will be compensated for by a layer of sodium and potassium ions.
The amount of positive and negative charge forming the alternating layers in the solution decreases as one moves from the negative protein nucleus towards the periphery of the system, until the random distribution of anions and cations once again prevails. Ultimately, to build an area at a different potential from its surroundings, it is sufficient to have a charge center formed, in which the positive or negative elements are immobilized (2 in Figure 3.4).
These compensation mechanisms make the system stable as the solution reaches equilibrium, even if there are differences in the microenvironments that make up the system. Differences in the number and concentration of electrical charges constitutes potential energy accumulations.
However, this system is not capable of transforming the accumulated energy into other forms of energy and ultimately into work because immediately after the introduction of the negative nucleus, a new stable equilibrium is instantaneously reached.
In order to have the possibility of accumulating potential energy and, at the same time, be able produce work, the system must be in a dynamic equilibrium between two or more interchangeable phases, in which the state differences are well-defined. In a system consisting mainly of water, these different phases will necessarily have to be differentiated by ionic concentrations.
Among the ions contained in seawater, where living organisms probably appeared, there is a high concentration of chloride and sodium and a relatively low concentration of potassium and calcium (Table 3.1).
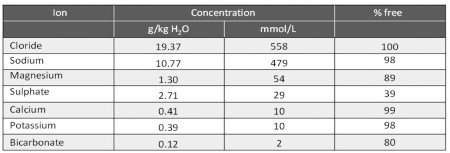
Table 3.1. Seawater composition (average salinity: 35‰)
If, in such an environment, it is necessary to create a concentration gradient between the negative protein nucleus and surrounding areas, it is essential that the nucleus has a high affinity for a specific ion, further reducing the system's degrees of freedom. The sodium ion, given its high concentration (1 in Figure 3.4), is not suitable for this purpose, as it would have to reach too high a concentration in the environment close to the protein core (2 in Figure 3.4).
Chloride is excluded, like sodium, because of its high concentration (Table 3.1) and the ionic situation close to the negative protein nucleus (a high concentration of fixed negative charges that functions to neutralize the charges of the cations). A significant increase in calcium ions is not feasible because it would compromise the biophysical characteristics of the system, as calcium compounds precipitate and form crystals, given their low solubility.
Therefore, the only ion suitable for accumulation near the protein core is potassium. If we assume that the negative protein nucleus, due to its biophysical characteristics, has a high probability of retaining potassium ions relative to other cations, the negative fixed charges will be neutralized mainly by this ion, which will form a first layer of cations (1 in Figure 3.5).
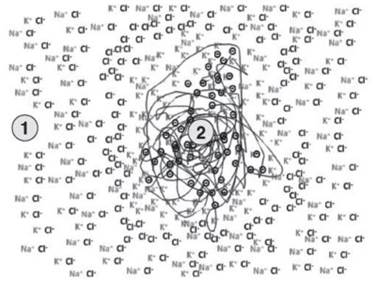
Figure 3.5. The selectivity for the potassium ion of the negative nucleus means that it is mainly this ion that neutralizes the charges (2). Going towards the periphery (1), the layers are randomly formed by the sodium, potassium and chloride ions
Moving away from the negative protein nucleus, layers of chloride ions and alternating layers of sodium and potassium ions will form. The layering becomes less and less evident due to the progressive decrease of the influence of the negatively charged nucleus. This continues until a random distribution of the ions typical of 1 in Figure 3.5 is again established. Also, in this case, the electro-neutrality of the system will be respected, if we consider sufficiently small micro-environments.
With the higher affinity of potassium ions for the negative nucleus, the ensemble of 1 and 2 are now two different environments with a real ion concentration difference, even if the whole system overall is in equilibrium. Potassium is more concentrated in 2, close to the protein nucleus, whereas sodium is practically absent (2 in Figure 3.5).
Sodium is more concentrated in the rest of the solution (1 in Figure 3.5). This system, however, does not have the possibility of modifying the distribution and the ionic concentration, except by modifying the consistency and the properties of the nucleus. Such a modification would require a lot of energy and relatively, a lot of time, as the synthesis of other proteins would be necessary.
Furthermore, although a difference in ion concentrations for each cation has been realized between environments 1 and 2 (Figure 3.5), the system is once again static and incapable of dynamically modifying itself and therefore of transforming the potential energy into work. It should be added that, despite local and small differences in electrical potential, in a system such as this, there is no real separation of charges.
Even where a transient accumulation of electrical charges would occur they would be immediately buffered by an ion with opposite charge to ensure the electro-neutrality of the solution.
Date added: 2024-07-02; views: 516;
