Methane Generation. Formation on CH4 by Methanogens
In most groundwater systems, detected methane concentrations do not approach the actionable level of 28mgL-1. In uncontaminated oxic aquifers, methane concentrations are generally below detection limits or at trace levels. However, methane is often found at measurable levels in aquifers with macro- or micro-environments that are sub-oxic to anoxic. Its concentration is often determined by a simple balance between multiple microbial reactions - those that produce methane (methanogens) and those that consume it (methanotrophs). Sources external to the groundwater system may add CH4, and there may be losses due to degassing and abiotic oxidative reactions. Figure 1 shows the main inputs, outputs, and controls on the final concentration of methane in a groundwater aquifer.
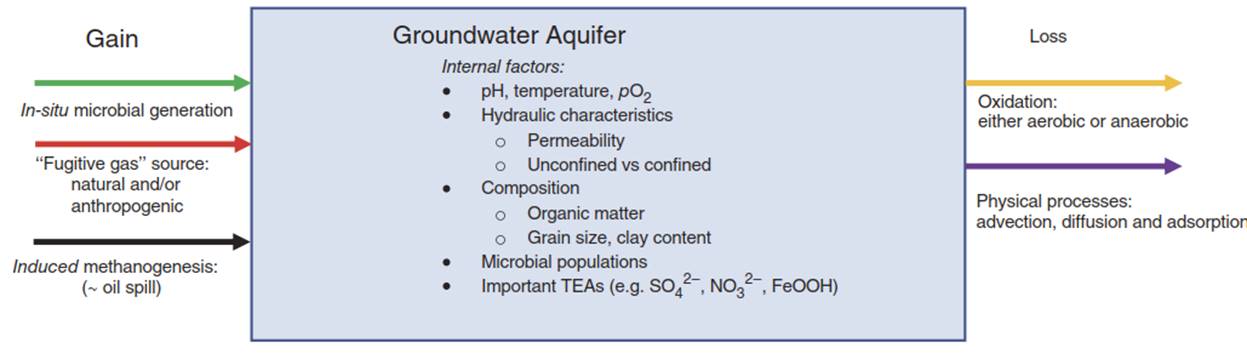
Figure 1. Schematic drawing of inputs and outputs to the groundwater methane budget. These are highly dependent on conditions within the aquifer as well as external sources. "TEA” is short for terminal electron acceptors, often the limiting nutrient for various microbial populations
Formation on CH4 by Methanogens. Methanogens are obligate anaerobes, requiring anoxic waters to live and produce methane. Many groundwater aquifers are anoxic due to the balance of O2 input (—atmosphere) and output, usually controlled by the quantity of reducible species available. Factors, such as length of flow path, organic content in the aquifer, or presence of reduced iron (Fe2+), manganese (Mn2+), or sulfide lead to O2 depletion. However, within an oxic aquifer, smaller “micro-environments" of anaerobic communities can exist.
All methanogens are Archaea that possess the unique ability to produce methane. While some suggest the existence of archaea that can produce ethane, herein, we will assume CH4 as the only gaseous hydrocarbon these microorganisms can produce. Types of substrates available to methanogens are extremely limited, often only the most simple compounds, such as acetate and methylamine (short-chained organic molecules), or CO2 and H2. Other microorganisms, specifically fermentative bacteria, provide the necessary precursors, both C-based and H2. Consequently, methanogenic activity, whether the carbon source is organic or inorganic, depends on a larger microbial community.
The two most common pathways that dominate CH4 production in nature are acetoclastic methanogenesis (the fermentative production of acetate):
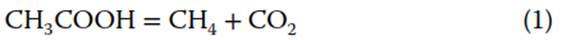
and hydrogenotrophic methanogenesis:
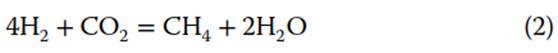
While early studies suggested that acetoclastic pathways were dominant in freshwater settings and hydrogenotrophic methanogenesis was more prevalent in marine environments, subsequent work attributes far more control to substrate availability rather than overall salinity. Waldron et al. showed activity by both types of archaea in shallow freshwater environments. In most instances, if a source of acetate is introduced, whether directly or indirectly by enhanced acetogenesis, the acetoclastic methanogenesis pathway will dominate and the microbial population will reflect this. In contrast, if acetate is limited and a source of H2 is present, hydrogenotrophic methanogenesis will dominate. The main control on the rate of methanogenesis via this pathway is the production of H2, whether by bacteria or inorganic processes such as serpentinization.
The rates of in situ production by either pathway is also dependent on temperature, nutrient availability, and the hydrologic system itself. The permeability of an aquifer and proximity to O2-rich water recharge are critical since even low O2 saturation levels will restrict CH4 generation. However, methanogens can tolerate some exposure to O2 in a dormant state. When conditions become anoxic, such as with a hydrocarbon spill, they will then proliferate rapidly.
The second most important factor limiting in situ methane generation, after O2, is competition with other anaerobic organisms for substrates. Where other terminal electron acceptors (TEAs) are present (such as Fe3+, SO42- Mn3+), iron, sulfate, and manganese reducers will “outcompete" methanogens for the H2 or acetate. This competition is governed by the relative amount of “energy" the organisms receive from each reaction. The order, often referred to as the redox ladder, places anaerobic microorganisms that use NO3-, Mn3+, Fe3+, and SO42- ahead of methanogens, no matter which pathway they are using to produce CH4.
The canonical view that methanogenic activity is determined by competition (defined by the “free energy" or AG of reactions) has recently come under some scrutiny. Bethke et al. have shown that under certain conditions sulfate reduction and methanogenesis can occur simultaneously. Similarly, methanogens that utilize a noncompetitive substrate (methylated compounds) are found at relatively high sulfate levels (~ 5 mM) in coalbeds.
In situ Consumption of Methane by Biotic and Abiotic Pathways. The major sink for methane in groundwater as well as in the atmosphere is oxidation. In the atmosphere, methane is mainly degraded by the OH radical, which is itself produced through photochemical reactions with oxygen. This breakdown of methane gives it a relatively short residence time in the atmosphere, ~9.6 years. In groundwater, both aerobic and anaerobic methanotrophs act as catalysts, greatly increasing the rate of degradation. In many sites of groundwater contamination, this oxic degradation of hydrocarbons has been well studied, with techniques such as air “sparging" used to mitigate the pollution. Methanotrophs also degrade methane via a series of anaerobic reactions. Depending on the concentration of other TEAs (e.g. SO42-, NO3-, Fe(OH)3) in the groundwater, methane can be consumed via the following reactions:
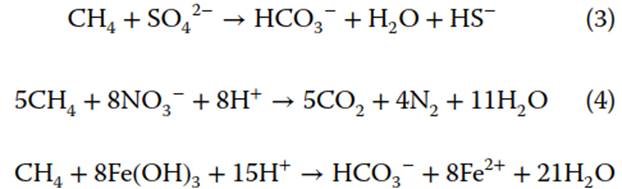
In addition, pathways that include manganese (Mn3+) and NO2- have been suggested, many relying on complex symbiotic interactions among microorganisms.
Summary. In-situ production of methane in groundwater is dominated by one of two pathways and dependent on a number of conditions within the reservoir. Chief among them is the presence of O2. In addition, even where anoxic conditions persist, competition from other anaerobic organisms keeps CH4 generation extremely low. Indeed, high concentrations of CH4 are suggestive of an external supply. Finally, methane concentrations are lowered by in-situ consumption of methane by methan- otrophs using various metabolites (e.g. O2, SO42-).
Date added: 2025-02-13; views: 280;
