Sorption to Coagulating Colloids and Aggregating Particles "Scavenging"
Coagulation, sorption, and uptake rates of COM onto particles are related to size and molecular weight distribution of DOC. Thus, one needs information from the application of not just one single method. For example, results from the application of state-of-the-art electrospray mass spectrometry techniques indicate average molecular weights of DOC, normalized to charge, of less than 1 kDa, likely due to fragmentation of carboxylic acid-rich compounds during ionization in a vacuum and acceleration in mass spectrometry.
On the other hand, much higher average molecular weights of 105 to higher than 106 Da can be estimated from images of freshwater or marine colloids when using AFM techniques of the same specimens mounted by adsorption or centrifugation onto mica surfaces that are flat on the atomic scale, consisting of abundant fibrillar macromolecules hundreds to thousands of nanometers long (Figure 2). Transmission electron microscopy (TEM) of heavy-metal-stained specimens embedded in hydrophilic resins confirms such estimates.
Colloids act as glues and soaps, as well as gels, helping in their capacity to aggregate carbon-containing particles. Thus, colloids become involved in a major way in the scavenging from the ocean waters of nutrients and pollutants, metals, radioisotopes, and, in short, any element in the periodic system, with sinking particle aggregates, all the while being degraded by bacteria. This overall scavenging process constitutes the “self-cleansing capacity" of aquatic systems. In addition to colloids interacting with each other or with larger particles, macromolecules of colloidal size also act as templates for building silica and carbonate shells of phytoplankton, i.e. biopolymers., and can contain important binding sites for radioactive and stable trace elements.
Thus, biopolymers control the scavenging of metals and radionuclides and the coagulation/flocculation of particles in marine systems, and references therein) and also the early development of biofilms, which is largely due to their surface-active nature. These biopolymers can also initiate or modify precipitation of MnO2 and FeOOH, SiO2, and CaCO3. Moreover, APS-rich polymers in the extracellular milieu form flocs and also bind extracellular enzymes in their active forms. The physicochemical properties of EPS molecules can also provide chelating sites for micronutrients such as trace metals. EPS can also modify the solubility and mobility of associated molecules. Other important chelating compounds are contained in microbially derived EPS that make up the bulk of what is called “marine snow" or “lake snow," and which in turn can also provide the organic “glue" for mineral aggregate buildup, thus providing a vehicle for transport to the seafloor (Figure 5).
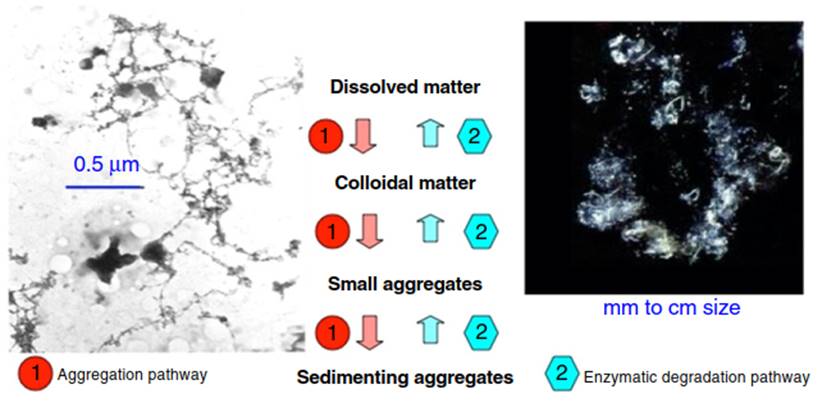
Figure 5. Owing to its sticky qualities, colloid aggregation (red) is a pathway that is opposite to the prevailing degradation pathway (blue) going from large to small molecules. This is illustrated by the (nm- to pm-sized) colloid "spiderweb-like” fibrils acting as "colloid-traps” (by Transmission Electron Microscopy (TEM), after staining with a heavy metal dye;, free access) on left, and centimeter-sized Marine Snow on the right. Marine Snow aggregates have fractal properties, i.e. showing similarities across scales, i.e. self-similarity and scaling invariance. Source: Alldredge and Gotschalk, with permission from the publisher
The colloid/particle partitioning is dynamic, as parts of colloids continuously aggregate to particles, while others disintegrate or decompose into smaller molecules through enzymatic and/or photochemical reactions. This coagulation or aggregation process counters a prevailing degradation pathway from young high-molecular-weight to older low-molecular-weight molecules (Figure 5).
Colloidal ligands that have surface-active properties and are found in the filter-passing fraction can be the cause of particle-concentration effects on particle-water partition coefficients (Kd, the concentration ratio in particles and in solution) and kinetic constants (ki) of trace metals or radionuclides, which have been documented in the literature for many years. Colloidal ligands can often be removed by coagulation and flocculation. Such particle-concentration effects can be eliminated or minimized, however, when one corrects for the presence of colloidal ligands.
234Th, which is generated in situ from the radioactive decay of 238U and has a half-life of 24 days, can be used to derive residence times of Th(lV)-binding colloids. Average colloidal residence times (with respect to coagulation) range from fractions of days in estuaries to a few weeks in the surface ocean. Similar results are obtained in controlled laboratory coagulation experiments using radioactive metals bound to estuarine COM. These laboratory results also confirmed particle-concentration effects on removal rate constants, previously demonstrated for Th(IV) in the laboratory and in the field.
Values of the particle-water coefficients (concentration in particles or colloids to that in water) of the A-metal 234Th(IV) to colloids (Kc) are generally similar to Kd values for particles [50], but this is not necessarily the case for B-metals such as Cu where generally Kc is larger than Kd. Aggregation rates of colloidally complexed metals show two distinctive reaction rates: a larger rate constant is consistent with Brownian “colloidal pumping" that occurs at a uniform rate and is faster at higher particle concentrations and a smaller rate constant that is different for different metals. What is assumed in this simplified “Brownian colloidal pumping model," as well as more sophisticated particle aggregation/coagulation models, is a constant physical (fudge) factor, a “sticky factor" (probability of sticking, with 100% = 1), with typical values of 0.1-1. Clearly, this factor contains coupled physical, chemical, and biological processes, which are simulated, to a variable degree, in some particle transport models.
Date added: 2025-02-13; views: 290;
