Development and Validation of Genetic Risk Scores for Cardiovascular Disease
One clinical application for cardiovascular genomics is the development of “genetic risk scores,” which can be used to estimate risk for CVD based on the presence or absence of multiple genetic variants. The development of such risk scores begins by defining the relationship between specific genetic variants and risk of CVD. For example, in the CARDIoGRAM plus C4D Consortium, investigators performed a GWAS meta-analysis from the 1000 Genomes Project, characterizing genetic variants in coronary artery disease (CAD) (evaluating 6.7 million common (minor allele frequency (MAF) >0.05) and 2.7 million low-frequency variants (0.005 < MAF < 0.05)).
The investigators confirmed the contribution of multiple known loci to CAD and also identified 10 new loci. The minor allele frequency of the implicated genes was high, which supports the common disease- common variant hypothesis. In another study involving an analysis of risk of coronary heart disease (CHD) in secondary and primary prevention trials (total of 48,427 individuals and 3477 events), patients were stratified by “genetic risk score” (defined by the presence of up to 27 SNPs, known to associate with CHD).
The authors defined low, intermediate, and high genetic risk, which predicted CHD events and statin benefit. The greatest odds ratio (OR) for CHD from the panel of SNPs came from SNPs in the gene for lipoprotein(a) (LPA): rs3798220 (OR 1.47) and rs10455872 (OR 1.70). After these SNPs in LPA, the SNP with next highest OR for CHD in this analysis was rs4977574 at the 9p21.3 locus (OR 1.29) (Fig. 4.1).
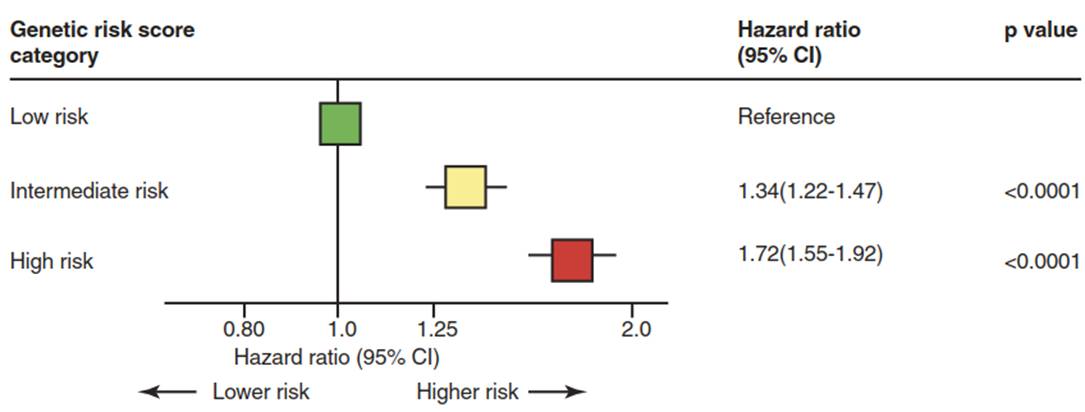
Fig. 4.1. An example of a genetic risk score used to estimate risk for coronary heart disease based on up to 27 single nucleotide polymorphisms (SNPs)
Another study examined the relationship between a CAD risk score (based on 50 SNPs) and cardiovascular outcomes in 3 large prospective cohorts. The investigators examined the interaction between genetic risk and lifestyle on outcomes and found that genetic risk can be offset by lifestyle. Another study evaluated a polygenic risk score based on 57 variants in the West of Scotland Coronary Prevention Study (WOSCOPS), Coronary Artery Risk Development in Young Adults (CARDIA), and Bioimage cohorts, and found that the greatest risk reduction from statins occurred in the subgroup with the highest genetic risk, despite similar degrees of low-density lipoprotein (LDL) lowering across genetic risk groups.
Also, investigators found that a higher genetic risk score was associated with increased coronary artery calcium and carotid artery plaque. A recent report describes the development and testing of a genome-wide polygenic score for CAD, including validation and testing. Here, the CAD risk score is based on >6 million variants and performed well as a risk estimator, and there was a large amount of patients (8%) who were estimated to be at >3-fold risk of CAD based on the CAD risk score; many more people affected than with FH.
It is likely that the predictive ability of such genetic risk scores will continue to improve as additional genetic variants implicated in CVD are discovered and incorporated into such polygenic risk estimates. As these polygenic risk estimates continue to improve, it is conceivable that their use will soon be translated into clinical practice and used alongside traditional risk factors (such as plasma lipid levels) to provide clinicians and patients with a more robust estimation of individualized cardiovascular risk.
Date added: 2025-02-17; views: 362;
