Diets for Captive Amphibians. Larval Amphibians
Introduction. Larval and adult amphibians consume very different diets in the wild than offered in captivity, and undoubtedly that is the etiology of many of the nutritional diseases known in captive amphibians. The prey species consumed in the wild is known for few species of amphibians, and from very small sample sizes of those species studied (e.g., Cornish et ah, 1995; DeBruyn et ah, 1996; Duellman & Lizana, 1994; Evans & Lampo, 1996; Tocque et ah, 1995). The nutrient composition of many actual or potential prey species are either incompletely studied (e.g., Reichle et ah, 1969) or virtually unknown—it is not feasible to scientifically formulate diets for captive amphibians based on the fieldwork done thus far. Analyses of species of some of the invertebrates and vertebrates used as food items have been compiled and reported (e.g., Dierenfeld & Barker, 1995), however these analyses are incomplete with regard to the levels of vitamin D3 and other nutrients.
When one considers the controversies that exist in the well-documented field of human nutrition, the long term suitability of a diet described for captive amphibians is difficult to assess. Fortunately many of the species commonly held in captivity adapt well to readily available food items, which may indeed be the reason why these species are common in captivity. Multigenerational breeding by more than one captive population is one of the few objective measures of success for a diet, and has occurred in only a few species (e.g., White’s treefrog, Pelodryas caerulea, the green and black poison dart frog, Dendrobates auratus, the dyeing poison frog, D. tinctorius, the African clawed frog, Xenopus laevis, the Cayenne caecilian, Typblonectes compressicauda, and the axolotl, Ambystoma mexicanum). Extensive field research and analysis of the nutrient composition of prey items and gastrointestinal contents is needed if the discipline of captive amphibian nutrition is ever to become a hard science.
Larval Amphibians. The diets fed to larval salamanders and neonatal caecilians are generally similar to those fed adults, although the items must be smaller in size. Tadpoles, however, may have diets radically different from adult anurans, for while adults are carnivorous many anu- ran species have tadpoles that are herbivorous or filter feeders. The tadpoles of some dendrobatids (e.g., strawberry poison dart frog, Dendrobates pumilio) are obligatorily oophagous and consume infertile eggs laid by their mother (Figure 6.1).
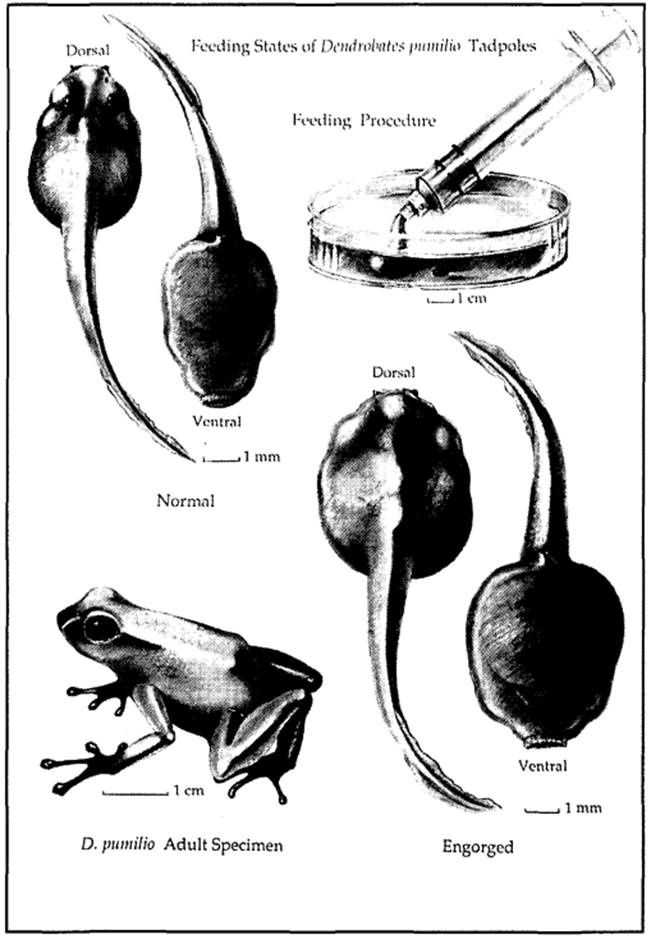
Figure 6.1. Obligatory oophagous tadpoles, such as the strawberry poison frog, Dendrobates pumilio, engorge themselves during feeding. Unfortunately attempts to rear these tadpoles on artificial diets as depicted here have met with minimal success (Heselhaus, 1992). (Caitlin Hughes)
According to one classification system based on the arrangement of the mouth and opercula, there are at least five types of anuran tadpoles (Duellman & Trueb, 1986; Orton, 1953; Sokol, 1975), and this oral structure is linked to the dietary preference. Some larval amphibians may have different morphologies depending on food availability, and may become cannibalistic in times of food shortages. This has been noted in the Plains spadefoot toad, Spea bombifrons, where some tadpoles have the typical scraping mouthpart while others develop an enlarged beak and jaw muscles and become predatory feeding on tadpoles (Bragg, 1965; Orton, 1954). A similar morphological range is noted in some populations of the tiger salamander, Ambystoma tigrinum. Some larvae develop much larger heads (and consequent mouth gape) and longer teeth than others and become cannibalistic (Rose & Armentrout, 1976). It is essential that one understand the life cycle of an anuran species when choosing a diet to feed the tadpole, or else malnutrition will result.
Carnivorous tadpoles, larval salamanders, and neonatal caecilians can be reared using a variety of whole and chopped invertebrates and vertebrates. Newly hatched larvae may be so small that fresh zooplankton netted from an unpolluted natural water source may be needed to establish feeding (e.g., Baker, 1988), although once they have reached a larger size other items can be used. Cultured protozoa (e.g., Paramecium spp.) and small crustaceans (e.g., Cyclops spp.) have been used as a food source for small larvae (Wisniewski & Pauli, 1983). Cultured rotifers are used extensively in raising fish fry and can be used as a food for larval salamanders. Other small crustaceans (e.g., Daphnia spp., newly hatched brine shrimp, Artemia salina) can be cultured as food sources.
Once the larvae have reached an appropriate size, generally over 10 mm (0.4 in) in length, larger food items may be offered. Some larvae have large enough mouths upon hatching to immediately take these larger food items. Small earthworms or chopped adult earthworms, bloodworms, glassworms, white worms, black worms, tubifex worms, mosquito larvae, small live freshwater fish, and chopped whole freshwater fish can be offered. Some larvae may learn to accept extruded, pelleted, or flake food designed for fish or reptiles (Baker, 1988). It is suggested that several different items are offered in the course of a week so that a varied diet can be consumed by the larvae. It should be noted that frozen zooplankton and other invertebrate food items are packaged and sold as fish food and are readily available at most pet stores that sell fish and fish supplies. The clinician is advised to determine local sources for these food items so that the client can quickly acquire food when the need arises.
Omnivorous tadpoles can be fed brands of flaked or pelleted food designed for omnivorous fish. The problem with many fish foods is that as the food particle size gets smaller, it has an increasing surface area-to- volume ratio which in turn increases the rate of leaching of water soluble nutrients. At least one study has documented that there is significant leaching of the water soluble vitamins from flaked foods, with up to a 90% reduction in cyanocobalamin (BI2) within 30 seconds of immersion in water (Pannevis & Earle, 1994). This loss of В vitamins appears responsible, at least in part, for the development of scoliosis, spindly leg, and paralysis of developing anurans. (See also Sections 7.8, Scoliosis; 7.9, Spindly Leg; 7.10, Paralysis; 18.1, Spindly Leg.) To minimize the loss of important nutrients, feed schedules should occur so that food is consumed immediately. Tadpoles that feed at the surface may be offered floating foods, while bottom feeding tadpoles may be offered sinking foods. It is often helpful to have live aquatic vegetation and (green) algae present in the water of omnivorous (and herbivorous) tadpoles as an alternate food source. One study documented the faster growth rate of tadpoles raised in a tank containing live algae, diatoms, and commercial food and supports the important role of good algal growth in tadpole growth (Kupferberg et al., 1994). Pood items suggested for carnivorous larvae may be offered sparingly to omnivores.
Herbivorous tadpoles can be fed flaked fish foods designed for herbivorous fish. Spirulina tablets are often used as a supplement to this diet with herbivorous tadpoles. As already mentioned, the presence of live aquatic vegetation and algae is suggested for good tadpole growth (Kupferberg et al., 1994). Blanched or microwaved romaine lettuce or other heat-treated greens can be offered in addition to the aquatic vegetation, but the produce should be replaced daily. Proper heat treatment of produce does not degrade the vitamins while it breaks down the structure so the tadpoles can easily graze upon it. Oxalate-containing vegetables such as kale and spinach are to be avoided as a food source to prevent renal disease from oxalate accumulation (National Research Council, 1974). Decorative plants such as the silver queen, Aglaonema roebelinii, may also contain calcium oxalate. Oxalate-containing plants should be considered a potential hazard due to the development of renal disease in some frogs at the National Aquarium in Baltimore held in enclosures containing silver queen, A. roebelinii (see Section 7.5, Renal Calculi).
Microencapsulated foods designed for filter-feeding invertebrates and fish fry have been used with some detritivorous tadpoles. However, better growth rates may be achieved by rearing the tadpoles in a tank containing a combination of algae, diatoms, and artificial food (Kupferberg, 1994).
There have been some studies documenting the effect of diet on growth of larvae and metamorphosis into normal adults (reviewed by Kaltenbach & Hagedorn, 1981), but a formula for success for a given species is still elusive. The efficacy of any diet is determined solely by trial and error, and evaluating the efficacy is incumbent upon standardization of other parameters such as water quality and temperature. Water quality should always be evaluated if growth rates of larvae are not optimal or if abnormalities of growth (i.e., delayed development) are noted. Furthermore, there may exist significant differences between the morphology, development, and behavior of the larvae of subspecies and geographic races within a single species (Wisniewski, 1992), and these differences may be erroneously interpreted as nutritionally related rather than having a genetic basis.
The effects of crowding or isolation, wherein the population density of the larvae within a tank is less than optimal, can also influence growth rates (Woodward, 1987), as can lack of appropriate cage furnishings (Wisniewski & Pauli, 1983). Inappropriate lighting may influence growth rates (Rugh, 1935), although this has received little attention since early studies. The frequency and predictability of cage servicing and feeding may delay metamorphosis and have other physiological effects, as was noted with the bullfrog, Rana catesbeiana, (Horseman et al., 1976). Some researchers have suggested that there are other growth inhibition agents that affect tadpole development, and the existence and exact nature of these agents have been debated (Beebee, 1995; Petranka, 1995). All these items must be considered whenever there appears to be a failure in development of larvae in order to determine if there is indeed a nutritional link.
Scoliosis and 100% mortality occurred in captive bred tadpoles of a phyllomedusine frog, Phyllomedusa cf tarsius, at the National Aquarium in Baltimore (B. Whitaker, personal communication, 1996), and high mortality from spindly leg was noted in the tadpoles of several species of dendrobatid frog (Epipedobates spp., Dendrobates spp., Phyllobates spp.). Successful rearing was achieved when vitamin В complex (Vitamin В Complex, VEDCO, Inc., St. Joseph, MO) was added to the tank water at a dose of 0.5 to 1 ml per gallon of tank water. One ml of this product contains 12.5 mg thiamine, 2 mg riboflavin, 5 mg pyridoxine hydrochloride, 12.5 mg niacinamide, 10 mg d-panthenol, and 5 pg cyanocobalamin.
New water used for water changes was supplemented at this level, and water changes occurred twice weekly. However, there was greater growth of algae in the supplemented tank than unsupplemented tanks, so the mechanism by which normal maturation occurred is unclear. Young specimens of the phantasmal poison frog, Epipedobates tricolor, were especially prone to spindly leg unless this rearing regimen was used. However, adding this vitamin complex to the water used at the Philadelphia Zoo did not prevent spindly leg in this species. The uncertainties associated with the В vitamin complex supplementation emphasizes the difficulty in evaluating larval diets for amphibians.
Date added: 2025-02-17; views: 402;
