Habitable Sites and the Evolution of Dispersal
Much of the conceptual framework of organism and environment can be consolidated into a model that interprets the distribution and abundance of organisms in terms of habitable sites. Aspects of the habitable site idea were developed mathematically by Gadgil (1971) and extended conceptually by Harper (1977, 1981b), among many others (e.g., Johnson and Gaines 1990; McPeek and Holt 1992; Travis and Dytham 1999; Levin et al. 2003; Ronce 2007).
What follows here builds on Gadgil’s notion and depicts it descriptively and graphically, rather than mathematically. In the ensuing decades since his conceptual paper on the evolution of dispersal, the issue has been explored in depth, as have the closely related topics of colonization, landscape heterogeneity, and metapopulation biology (see e.g., Hanski and Gilpin 1997; Turner et al. 2001), as well as the life history correlates of dispersal (Stevens et al. 2012). With some exceptions (e.g., Andrews et al. 1987; Taylor and Buckling 2010) this body of theory has not been integrated into microbial ecology (or vice versa), where the focus has been on bacterial and fungal dispersal, host range of pathogens, cropping patterns, and epidemiology (Baker and Cook 1974; Burdon 1987a).
Habitable space can be defined as a zone in which biotic and abiotic conditions allow an organism to become established and survive competitively. Obviously the clearest example of a habitable site is the place where an organism can complete its life cycle (i.e., including a reproductive phase). A site is not habitable to a species simply because it contains viable propagules, such as seeds or spores, of that organism. Habitable sites are thus distinct from ‘sink’ sites, which may contain individuals—perhaps even consistently and in relatively high numbers—but only because they are supplied as immigrants from a source site nearby (Pulliam 1988).
The human colon but not a soil particle is a habitable site for E. coli. Thus, habitable space consists of localized, favorable conditions that occur as islands or patches within a biologically and physically hostile sea. Distribution and abundance will then be determined in large part by the match between characteristics of the organism (e.g., dispersal ability; intrinsic population growth rate) and environmental pattern (e.g., size, number, distance between, duration, and carrying capacity of habitable sites). As seen in the preceding sections, chances for a match are improved by phenotypic plasticity of individual colonizers, and by genotypic variation within the colonizing population to cope with the variability of sites. Occasionally, habitable sites used alternately by a particular colonist, can have very different characteristics (see Sidebar, A Case Study).
Habitable sites also have variable temporal and spatial components. This is perhaps best illustrated by considering how environments could appear in time and space to an organism (Southwood 1977). With respect to time they can be: (i) constant (favorable or unfavorable indefinitely); (ii) predictably seasonal; (iii) unpredictable; or (iv) ephemeral (predictably short, favorable conditions followed by unfavorable conditions for an indefinite time). In space, habitats can be: (i) continuous (favorable area exceeds area that organism can reach regardless of dispersal mechanism); (ii) patchy (unfavorable areas surrounding favorable islands which can be reached by dispersal); or (iii) isolated (favorable but unreachable). Consider a migratory bird that breeds over the summer in northern Canada and passes the winter in the southern United States.
The two sites are predictably seasonal and the bird’s life cycle is adjusted to exploit both. On a local scale, the relative area occupied at each site and population density will fluctuate annually depending on factors such as pressure from competitors and predators, which also occupy the site. The life cycles of plant and certain animal pathogens that alternate habitats is directly analogous. The remarkable intersection in time and space between habitable sites and appropriate growth stage to colonize them is best seen among species with complex life cycles (Chap. 6; see also Chap. 3 in Price 1980). So, sites are highly plastic in size, may be temporally discontinuous, and are not species-specific. They can be depicted simplistically in three-dimensional form through time as meandering tubes of irregular diameter (Fig. 7.8). Individuals in contemporary time or lineages in evolutionary time can escape extinction by surviving competitively in a particular habitable site, reproducing sexually or asexually, and then dispersing to a former or different habitable site.
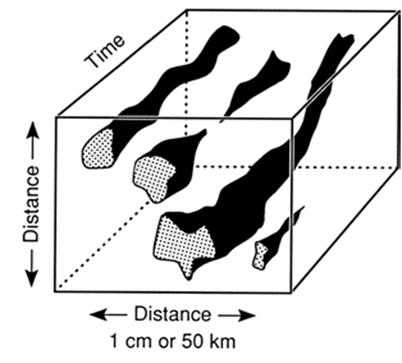
Fig. 7.8. The habitable space concept. Favorable sites, projected through time, appear as continuous or discontinuous patches (irregular tubes). Species cohabiting a site may or may not directly interact. In response to biotic or abiotic factors, any given species may increase, become temporarily dormant, or decline possibly to local extinction pending recolonization
The dynamic aspect of habitable space results not only from changes in the organism or the site, but also from interactions between organism and site. It was noted at the outset of the chapter that, strictly speaking, separation between organism and environment is impossible. As the organism moves through its life cycle it selects those parts of the site that are relevant to itself (Lewontin 1983) and reorganizes them: Twigs become nesting material for birds; trees are felled to become food and shelter and dams for beavers; lignin and cellulose in wood are degraded by fungi and the monomers incorporated into fungal protoplasm while fungal metabolites are also extruded and further modify the habitat and influence succession (Lonsdale et al. 2008); the DNA of a plant host is reprogrammed by a tumor-inducing plasmid of the crown gall bacterium to encode unique molecules (opalines and nopalines) used as nutrients by the pathogen and not the host (Chap. 3 and Platt et al. 2014).
Organisms modify a site further by such activities, in the process often making it more-or-less habitable by certain other species. In the extreme case, a successional sequence develops, as consortia of organisms are displaced by successors better adjusted to the dynamically evolving environment-community matrix (Weiher and Keddy 1999). A classic example is the loss of a major ‘foundation species’, chestnut, from the southern Appalachians where it has been a co-dominant with oak for thousands of years, as well as in the Northeast (Paillet 2002). This was caused by the introduction from Asia of a virulent fungal pathogen in the early 1900s. The result was both the devastation of an entire tree species throughout its range and an entirely changed forest ecosystem. This is but one example among many of the far-reaching ramifications surrounding loss of dominant, influential species (for examples and terminology see Ellison et al. 2005).
Distance between habitable sites is arbitrary and related to distribution of favorable conditions relative to the size and growth form of the organism, which in turn determine other significant correlated attributes. For instance, colonizable patches may be separated by distance on the order of micrometers for bacteria or yeasts growing on a leaf surface; by meters for earthworms; or by up to kilometers for the Glanville fritillary butterfly in the Aland Islands of Finland. That habitable space may exist unfilled is clear from the frequent success of intentional species introductions or inadvertent invasions (Mooney and Drake 1986).
Distance between real or virtual islands is a factor driving dispersal and molding life cycle features. The probability of colonizing any site is a function of several factors, among them the probability of propagule survival and the number of arriving propagules. Gadgil’s (1971) models show that, as a result of dispersal, isolated, poorly accessible sites should be less crowded than an average site. A single episode of dispersal can produce appreciable differences in the extent of crowding at the various sites. A phenomenon that might be called ‘immigrant amplification’ can occur in which, even though dispersal results in no net additions or losses of individuals, it can still increase mean population densities if the episodes of net migration are positive when populations are growing and negative when they are declining (Ives et al. 2004). When migrants have high rates of mortality, the dispersal event could reduce crowding at all sites, effectively lowering the total population size of the species. Likewise, the greater the variation in carrying capacity among the different sites, the greater the impact on over- or undercrowding. This will tend to depress the metapopulation size over its entire range.
Species have different tendencies to disperse and different dispersal characteristics. This will affect gene flow (used here to mean gene movement among different populations of the same species). For instance, dispersal is generally strong and rigidly programmed in insects, evidently as an adaptive response to their short life cycles and ephemeral breeding sites. Migration is less programmed in vertebrates, but still predictable based on certain factors such as sex and age (e.g., young males are the most prone to disperse in baboon troops and lion prides; Wilson 1975). Plant seeds and microbes even more so are the ultimate nomads; the old adage “everything is everywhere, the environment selects” is commonly applied to microbial biogeography and powers of dispersal.
Historical, geographical, or geological constraints are less important considerations in assessments of distributional patterns than they are for macro organisms (Chap. 4 in Brock 1966; Hanson et al. 2012). Small size facilitates long distance movement by wind, water, or vectors across barriers which would be insurmountable for macroorganisms. A short generation time and high fecundity, which imply potentially high population densities, favor colonization. Fungi particularly seem to be close counterparts to insects in programmed dispersal characteristics (timing in life cycle; specialized migratory forms), although there are obvious differences in that fungal dispersal is largely passive, whereas insect dispersal is frequently directed. Dispersal characteristics and colonization dynamics have been examined at length elsewhere (MacArthur and Wilson 1967; MacArthur 1972). Where dispersal is passive, as is typical in plants and microbes, the number of individuals declines exponentially (e-x) with distance x from the source. Where dispersal is directed, as in the case of many animal search patterns, the decline follows a normal distribution pattern (Wilson 1975, pp. 104-105). The two dispersal types will thus have very different effects on the rates of gene flow.
How has dispersal evolved? Since travel is risky, migration will only be favored by natural selection if the chances for finding a better site exceed those of colonizing a worse site and of death in transit. A strategy of partial population migration may be the fittest (Gadgil 1971). Harper (1977) has added that for plant seeds it may occasionally be better to remain dormant indefinitely in situ without dispersal. A case would be where there is a higher probability of a conducive environment reappearing that that of dissemination to a distant habitable site. In general, however, dispersal must be advantageous because it is exhibited in some form in all phyla and is typically marked by adaptations that promote survival and dissemination. Dispersal can also entail considerable cost to the organism in expended time and resources.
Birds migrate only after physiological preparation and training flights, and lose massive amounts of stored energy reserves en route. In the slime mold Dictyostelium, up to 90% of the formerly free-living cells are assigned to act as stalk cells to elevate the fruiting body. They die as they are incorporated into the stems (Whittingham and Raper 1960), so the benefits of getting spores to a favorable ‘launch site’ and a new, potentially habitable site must outweigh these costs (Bonner 1982a). The benefit of dispersal to the individual (as opposed to the weaker argument for group selection at the species level) is the chance to colonize an empty or rarefied habitat, and the initial mating advantage over the locals upon entering the new population (part of the process of so-called ‘migrant selection’; Wilson 1975).
In many if not most unicellular organisms, dispersal may be incidental and the cells involved essentially no different from those remaining at the site of production. Incipient forms of dispersal were likely rudimentary, haphazard processes. It is easy to see how chances for successful migration could be improved by modest physiological changes, such as evolution of a shut-down phase (Lennon and Jones 2011), or by cells that became less adherent than their counterparts. The population would thus become partitioned into members suited for active growth or for survival and transport. Among bacteria, several extant species, such as among the myxobacteria and Caulobacter spp. discussed previously, have stages in their life cycles morphologically adapted for dispersal. This is not unlike the segregation of insect populations into sedentary and mobile forms (winged versus non-winged aphids; solitaria and gregaria phases of the African locust).
A later evolutionary development than simply shut-down, perfected by the fungi, would have involved cells (spores) packaged for transport with a nutrient reserve and thick walls and, in many cases, propelled on their way by elaborate release mechanisms (Ingold 1971). Wind dispersal of bacteria, fungal spores, and pollen has been studied for decades since the classic volume of Gregory (1961) on aerobiology. The importance of dispersal for the fungi is strikingly revealed in the elaborate adaptations for spore production, discharge, and dispersal. This includes the shape of the mushroom fruiting body (including the bell-shaped cap and height of the stalk), mechanisms to forcibly discharge spores or promote entrainment in air currents, and the evolution of spore shape to minimize drag (Deering et al. 2001; Trail 2007; Roper et al. 2008, 2010).
In plants and fungi there is obviously a close linkage between dispersal and sexual reproduction. Possibly selection acting on the latter led to adaptations also fostering the dispersal process. The container enclosing the products of meiosis, such as pollen, or of genetic recombination such as seeds, sexual spores, cysts, might have been an easily transported unit (Bonner 1958). Organisms reproducing largely by clonal means in effect sample new environments by growth (Jackson et al. 1985; Hughes 1989). It was noted previously (Chaps. 5 and 6) that while such organisms as strawberries and corals can in theory grow in unlimited fashion, in practice it is only a matter of time before clonal individuals reach the boundaries of their habitable sites (Chap. 3 in Williams 1975). Dispersal by clonal fragmentation is merely an extension of the growth process whereby the genet can move farther afield, freed from the handicaps related to physiological transport and allocation problems, or systemic toxins and infections, of operating as a single, massive physiological unit. Mobile, unitary organisms typically select sites in an active, controlled manner, whether this be accomplished by relatively primitive chemotaxis mechanisms (nematodes with their plant hosts), or by sophisticated olfactory, visual, and acoustic cues (vertebrates and higher invertebrates). Hence, a fundamental distinction in dispersal seems to be drawn along lines of growth form (Chap. 5), rather than kingdom.
Whatever its origin, dispersal is likely best interpreted within the context of challenge and opportunity met gradually and improved upon over geological time by different organisms interacting with their environments. This returns us to the opening remarks of the chapter: In evolutionary terms it seems no more likely that movement by one means or another to habitable sites suddenly became a ‘problem’ to be solved by terrestrial species than did swimming in water become a ‘problem’ that seals ‘solved’ by losing their legs. Lewontin (1983) observes that seals developed flippers and in so doing likely incorporated water as a progressively greater component of their environment. Analogously, almost from the inception of life, a migratory phase joined a sedentary phase as an increasingly important and programmed component of the life cycle.
Date added: 2025-06-15; views: 258;
