Phenotypic Plasticity in Action: Environmental Shape-Shifting in Plants, Fungi, and Microbes
Plants. Aquatic or desert plants in particular are noted for their conspicuous phenotypic plasticity (Bradshaw 1965; Valladares et al. 2007; Nicotra et al. 2010), perhaps most strikingly shown by environmentally determined leaf form called heterophylly (Fig. 7.5 and Hutchinson 1975, pp. 157-196; Bradshaw 1965). For instance, a single aquatic plant may bear two or more kinds of leaf, typically a dissected form in the water column and a laminar form above it. The finely dissected leaves increase area-to-volume ratio. This means that tissues are more accessible to dissolved CO2 for photosynthesis underwater (Hutchinson 1975, pp. 146-148). The triggering factor, depending on species, may be the prevailing conditions such as water level; in other cases it may be a seasonal cue such as temperature, photoperiod, or light intensity when the leaf is initiated. The molecular mechanisms underpinning heterophylly are not known in detail. The developmental switch in one case appears to be the regulation of the hormone gibberellin by expression levels of homeobox KNOX1 genes (Nakayama et al. 2014).
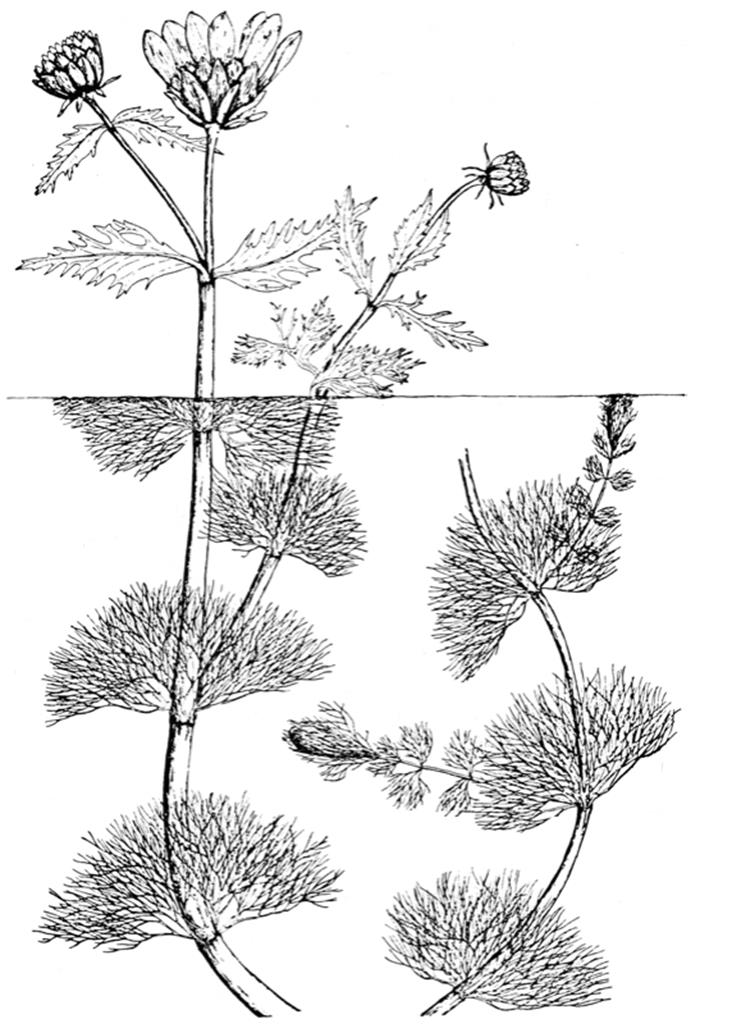
Fig. 7.5. The water marigold (Megalodonta beckii) exhibits a type of phenotypic plasticity known as het- erophylly, illustrated by the broad aerial leaves and the finely dissected leaves below the water line. Dissection of the leaf lamina enhances the rate of photosynthesis underwater by increasing the area:volume ratio, which increases CO2 assimilation from the surrounding water. Note that this rooted aquatic plant occupies three environments: sedimentary, aqueous, and aerial. This type of environmental polymorphism is controlled by a condition-sensitive switch (West-Eberhard 1989; Nakayama et al. 2014). From Fassett (1957), reproduced by permission of the University of Wisconsin Press, ©1957
Dimorphism among desert plants is related to water economy (Orshan 1963; Westman 1981). Plasticity is also apparent in the resource allocation patterns common to all plants, either to vegetative biomass for foraging purposes, or to reproduction, depending on the prevailing environment (Abrahamson and Caswell 1982; Grime et al. 1986; Bazzaz et al. 1987). Other common manifestations of the enormous morphological plasticity of plants include the ‘shade avoidance syndrome’ of shade-intolerant plants (Pierik and de Wit 2014) and the variation among flowers of the same individual (e.g., adaptations of some plants for open pollination in early-season flowers versus adaptation by way of closed flowers produced later to ensure self-pollination).
How do plants sense and respond to environmental variation? A striking example is the report by Braam and Davis (1990) that Arabidopsis can detect numerous experimental stimuli such as sprayed water, touch, sub-irrigation, wind, darkness, and wounding. The expression of at least four touch-induced (TCH) genes is affected by these stimuli and, additionally, growth is inhibited by touch. The touched plants have shorter petioles, begin to bolt later, and develop shorter bolts. This implies that plants can detect, transduce, and respond to environmental signals such as wind and rain, as well as mechanical loading and above-or belowground obstacles.
Interestingly, subsequent work shows that the TCH genes are inducible by nonmechanical stimuli such as darkness and extreme temperatures (Braam 2005; see also Monshausen and Haswell 2013). The growth response in Arabidopsis is gradual and differs from the immediate morphological reaction of such plants as the Venus fly trap (Dionaea muscipula) or the sensitive plant (Mimosa pudica). Nevertheless, Arabidopsis responds very quickly at the molecular level (within 10-30 min the amount of mRNA increases up to 100-fold). Three of the TCH genes encode calmodulin and related proteins, which implies that Ca+2 is involved in signal transduction and response. The fourth is a cell-wall modifying enzyme, a glucosylase/hydrolase. Other molecules implicated in the signaling network include reactive oxygen species, ethylene, and octadecanoids. Braam (2005) showed by gene-expression profiles that there are 589 genes or more than 2.5% of the Arabidopsis genome upregulated at least twofold in expression within 30 min of touch stimulation. Among the most highly represented functional classes involved are genes involved in defense responses. Recent evidence suggests that plants can respond systemically with chemical defenses to leaf vibrations induced by the feeding activity of insects (Appel and Cocroft 2014). Thus, we now have the foundation for a mechanistic interpretation as to how plants may be able to react rapidly and sensitively to environmental cues.
Microorganisms. To what extent are microbes phenotypically plastic? Indeed, they are extraordinarily malleable though one might not draw this conclusion because much of the relevant literature is presented in molecular or physiological semantics in the specialist journals, not within the conventional ecology mainstream. While most attention to bacterial evolutionary ecology has been paid to their genetic dynamics (Chap. 2), phenotypic plasticity is probably as important or arguably more so to their adaptive success. Bacterial plasticity is expressed mainly at the physiological level illustrated by an ability to adjust growth optimally as conditions warrant (previous discussion above in Sect. 7.4 and Chap. 3). One way bacteria do this is by producing spontaneously and reversibly a slow-growing cohort of the population that is resistant to unfavorable conditions such as the presence of antibiotics (Kussell and Leibler 2005; Helaine and Kugelberg 2014; see discussion of bistability, bet-hedging, and the bacterial persister phenomenon under Dormancy, later). Similar switching mechanisms are also common in the fungi and slime molds (below).
Another bacterial metabolic tactic is to alter the structure of certain macromolecules for metabolic optimization. This can be important in habitats where nutrient conditions are not simply always constraining but rather fluctuate irregularly from non-limiting to limiting. The case of adaptation to S-limitation by cyanobacteria is instructive. These bacteria are photoautotrophs (Chap. 3) that harvest light with chromophoric proteins called phycobiliproteins in a manner not too dissimilar from plants (Madigan et al. 2015). Such protein complexes may account for 60% of the soluble proteins of cyanobacterial cells, with sulfur represented in the amino acids cysteine and methionine. It follows that the phycobilisome proteins would be a likely target for selection pressure to economize cellular sulfur to avoid impairment of protein synthesis in habitats where that element is periodically limiting.
Mazel and Marliere (1989) showed that Calothrix compensates in such situations by encoding S-depleted versions of its most abundant phycobili- some proteins, which it specifically expresses under S-deprivation. Remarkably, the bacterium in effect carries the genetic equipment (operons) for two complete, functional sets of these proteins—one set containing the sulfur amino acids as usual and the other the methionine and cysteine-depleted set (for background information and analogous examples, see Merchant and Helmann 2012; Flynn et al. 2014). In habitats such as these where a resource supply oscillates erratically between sufficiency and insufficiency, it can make better adaptive sense to carry the capability to function in both circumstances rather than rely on mutation to provide the requisite set whenever it is needed. (In a similar context, Levins [1968, pp. 11-12] discusses potential adaptive strategies for bacteria carrying an inducible enzyme under various scenarios.)
Being of relatively simple and more-or-less fixed shape (Young 2006), bacteria do not appear to vary growth form as dramatically as do the fungi (below), though there are exceptions. For example, the bacillary shape of E. coli cells can become filamentous, a transition that is adaptive in uropathogenic strains as the latter phenotype resists phagocytosis (Justice et al. 2008). An initial, transient, resistant response to the antibiotic ciprofloxacin, also involving E. coli cells, is formation of extensive filaments containing multiple chromosomes (Bos et al. 2015). Bacterial filamentation, long considered to be a ‘sick’ phenotype, is increasingly viewed as an adaptive response at least in some situations. A more subtle but ecologically and medically critical modification is the difference in external structure between attached and planktonic cells of biofilm-forming bacteria (Costerton et al. 1995; Hall-Stoodley et al. 2004; for mechanisms underpinning sessile versus motile phenotypes, see Norman et al. 2013). The two cell types are functionally distinct though genetically identical, as is the case for differentiation of cell types within a biofilm in response to environmental as well as cell-cell signals (Vlamakis et al. 2013).
Furthermore, there are a few extraordinary instances of morphological plasticity among bacteria. The myxobacteria (Fig. 7.6), noted previously (7Chap. 4), are fundamentally single- celled, but manifest multiple phenotypic forms, changing among them based on intracellular and extracellular signals (Goldman et al. 2006; Madigan et al. 2015). They inhabit mainly topsoil but also diverse other interesting sites such as the bark of some trees (Reichenbach 1993). Individual cells move by gliding and can aggregate into swarms and mounds. This stage typically progresses eventually to a multicellular, differentiated fruiting body of varying, species-specific complexity, within which some cells form myxospores that germinate to produce vegetative cells.
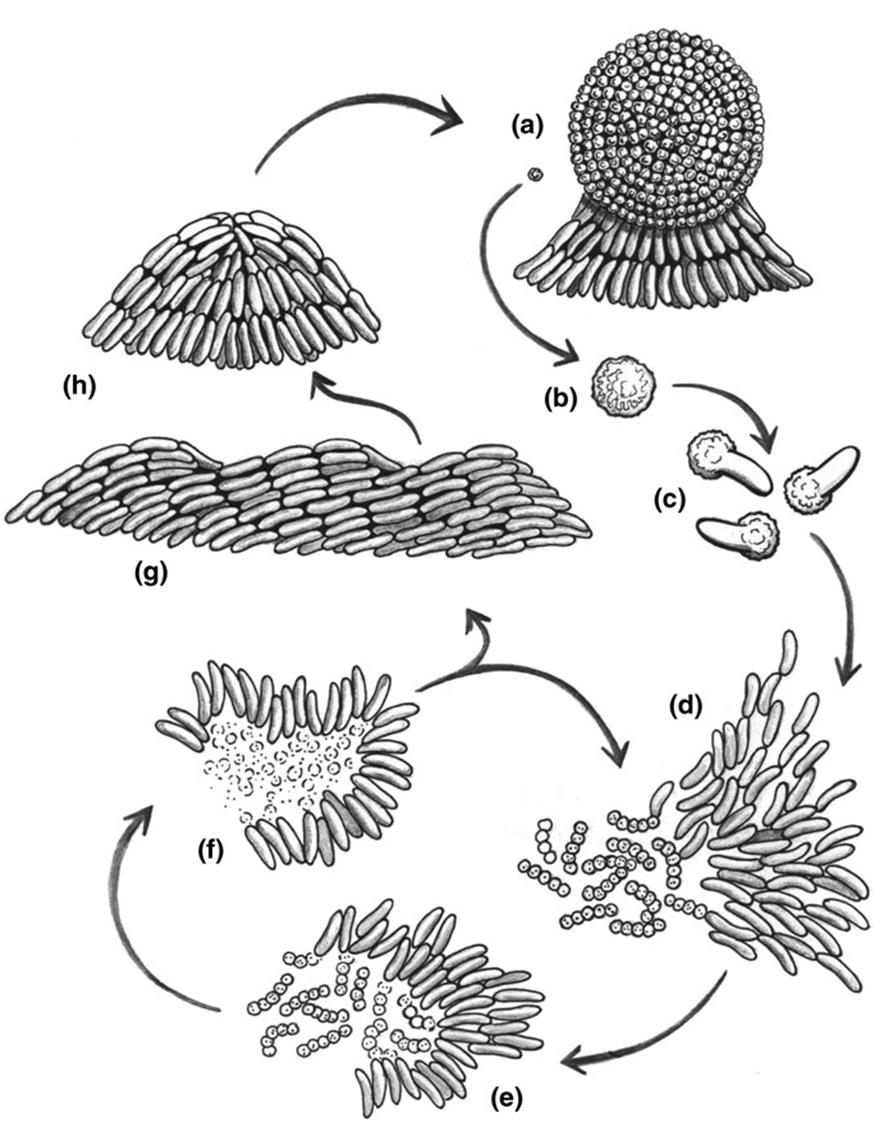
Fig. 7.6. The life cycle of Myxococcusxanthus, a predatory, social, soil-dwelling bacterium, shown here engulfing micrococci (chains of spherical cells in d, e). It transitions among various phenotypically plastic morphological states by integrating many cues from its external environment with physiological signals from within. Nutrient limitation triggers cellular aggregation (g), fruiting body development (h), and ultimately cell differentiation and sporulation (a, b). Nutrient sufficiency signals spore germination (c) with cells congregating into a feeding 'swarm' (d) that moves en masse by gliding, surrounding (d, e) and digesting (f) prey cells. Cooperative interactions are orchestrated by cell-to-cell signaling. From Goldman et al. (2006). Reproduced from Proceedings of the National Academy of Sciences, by permission of the National Academy of Sciences ©2006
The fruiting bodies are macroscopically visible, often brightly colored, and may consist of a billion cells. The onset of these and the various social aggregates is controlled mainly by environmental nutrient status, to which the bacterial cells respond by cell-to-cell exchange of soluble signals likely involving quorum-sensing or chemotactic mechanisms. These organisms present a fascinating example of an entity at the threshold of multicellularity and ‘collective individuality’ in its numerous phenotypic states from unicellular to multicellular, and prompt introspection as to how such lifestyles have evolved. Sequencing of the M. xanthus genome shows it to be comparatively large (9.14 Mb), an increase of some 4-5 Mb relative to other sequenced relatives (8-proteobacteria) (Goldman et al. 2006).
After ruling out other possibilities, the authors suggest that gene duplication and divergence were major contributors to separation from the most recent common ancestor; moreover, the genes were not duplicated at random but at specific sites such as for cell-cell signaling and small-molecule sensing. These social bacteria represent evidently a very successful evolutionary experiment as judged by the large numbers of known myxobacterial species (~50), high densities of cells (millions) per gram of many kinds of soils, and worldwide distribution (Reichenbach 1993; Kaiser 2003; Goldman et al. 2006).
Fungi. These organisms have shorter generation times than plants and are composed of fewer cell types (Chap. 4), all of which are modifications of the basic hyphal unit. Not surprisingly, then, the fungi are even more plastic than plants (see e.g., Jennings and Rayner 1984; Gow and Gadd 1995). Some may generate complex organs such as rhizo- morphs from diffuse or compact mycelium, which is itself variable in many features such as internode length, branch angle, and growth pattern (Chap. 5 and Cooke and Rayner 1984; Rayner 1991; Andrews 1991, 1995; Riquelme et al. 2011). Some organisms can alternate between a harmless, commensal state and a virulent, pathogenic condition depending on the environment.
Certain fungi take this to the extreme by undergoing a dimorphic transition, changing from a filamentous (hyphal) form typically found in an environmental reservoir such as soil or vegetation, to a single-celled (yeast) state in an infected human host (Nemecek et al. 2006; Klein and Tebbets 2007). The hallmark environmental trigger for this phase transition is temperature (e.g., Histoplasma capsulatum grows in a mycelial form at 25C, but as yeast at 37C), although other host factors such as nutrients and hormones are to varying degrees involved.
This extreme phenotypic shift is associated with differential expression of hundreds of genes; it is concurrent with and may be mandatory for virulence (Klein and Tebbets 2007, but see Sudbery 2011). Some of the human pathogens are actually trimorphic, having the capacity to grow as yeasts and hyphae as well as an intermediate form, pseudohyphae (Bastidas and Heitman 2009; see Carlisle et al. 2009 for morphological differences between pseudohyphae and hyphae, and role of gene expression in morphological switching). In Candida albicans, for example, the yeast phase is characteristic of its benign life as a commensal associated with the human mucosa, skin, and the gastrointestinal tract. However, in the pathogenic state of the fungus, the yeast form is adapted for systemic dissemination within the host, while the hyphae are responsible for infection and death of host cells (Bastidas and Heitman 2009).
The role of pseudohyphae remains controversial as is the evolutionary origin and development of the dimorphic phenomenon. It is notable that some nonpathogenic fungi, such as the common yeast Saccharomyces cerevisiae, can display a dimorphic (yeast to pseudohyphae) state that tends to be overlooked because it is unique to certain strains and culture conditions (Gimeno et al. 1992). In Saccharomyces, pseudohyphae are triggered by nitrogen limitation and may be adaptive in nature, enabling the wild yeast to forage for nutrients by expanding radially thereby advancing into uncolonized habitat (Gimeno et al. 1992; see also Bruckner and Mosch 2012) (Fig. 7.7).
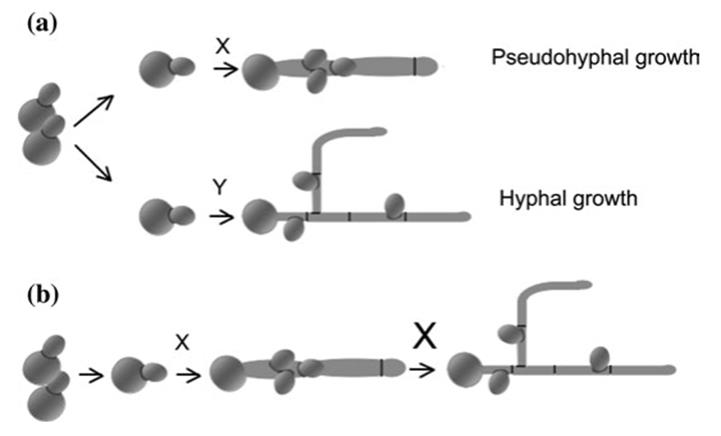
Fig. 7.7. Phenotypic plasticity as represented by morphological transitions of dimorphic or trimorphic yeasts represented by the commensal and human pathogen Candida albicans. The fungus can undergo dimorphic transitions from budding cells to either hyphae (a) or an extended, intermediate form known as pseudohy- phae (also a) under the control of different factors (X, Y). In (b) there is continuous transition from yeast to pseudohyphae to hyphae triggered by a dosage-dependent factor (x, X). For elaboration of this model, see Carlisle et al. (2009). From Bastidas and Heitman (2009). Reproduced from Proceedings of the National Academy of Sciences, by permission of the National Academy of Sciences ©2009
Unfortunately, relatively little is known about the ecology of the many human pathogenic fungi that spend most of their life histories in environmental reservoirs and for which association with a human host is largely accidental. Attention has focused instead on clinical aspects. Many if not all of these fungi probably grow as commensals or saprophytes of living or dead plant matter or other biomass and have adapted only secondarily as pathogens.
Date added: 2025-06-15; views: 261;
