Phenotypic Plasticity: Classifications, Adaptive Value, and Evolutionary Constraints
Any kind of environmentally induced phenotypic variation in behavior, physiology, or morphology is termed phenotypic plasticity (Stearns 1982; West-Eberhard 1989; DeWitt and Scheiner 2004). When the phenotype varies as a continuous function of an environmental parameter the relationship is known as a reaction norm. Such changes can be complex functions of the environment and may involve delays in expression, reversibility, and occur at different developmental stages, in addition to wide variation in degree of expression (Schli- chting and Pigliucci 1998; Givnish 2002). The topic as well as the biological phenomena underlying it are indeed vast as West-Eberhard (1989) has emphasized—‘phenotype’ including all aspects of an organism other than genotype, and ‘environment’ including both the external surroundings as well as the internal cellular milieu that influences gene expression.
Piersma and Drent (2003) subdivide phenotypic plasticity into developmental plasticity, polyphenism, phenotypic flexibility, and life cycle staging, expanded briefly below (see also Table 7.2 with the caveat that terminology varies in the literature and is used inconsistently). The table and examples cited give some impression of the pervasiveness and range in plasticity possible.
Developmental plasticity refers to the irreversible variation in some trait(s) arising during development in response to one or more environmental signals. For example, the acorn barnacle Chthamalus anisopoma responds developmentally by changing the architecture of its external calcareous plates in the presence of a snail predator (Lively 1986). Dramatic changes in phenotype as a result of morphogenesis are also seen in plants, many benthic marine invertebrates, and the holometabolous insects (where the same genetic entity may at one moment be a caterpillar and then next a butterfly). Some authors, however, consider such varied forms produced in standardized, ontogenetic sequence to be plasticity and others do not.
Polyphenism is the ability of an organism to change developmentally but in a seasonally cyclic fashion (Moran 1992). An extraordinary example is developmental polymorphism in caterpillars of a geometrid moth of the southern US and northern Mexico (Greene 1989). The spring brood mimics oak catkins on which they feed; the summer brood develops into mimics of oak twigs. This switch is triggered evidently by diet, as all caterpillars fed experimentally on catkins, which are low in tannin, developed into catkin morphs whereas those raised on leaves, which are high in tannins, resembled twigs. How such striking morphological responses at the organism level result from a cascade of physiological and genetic reactions at the subcellular level, initiated by an environmental signal, is a fascinating question.
Phenotypic flexibility refers to the noncyclic, potentially reversible variation within a single individual in behavior, physiology, or morphology in response to environmental signals. Although sea urchins have a hard exoskeleton, they are able to change body size by positive or negative growth in response to food abundance (Levitan 1989); plants, because of their modular nature, are highly morphologically plastic (Chap. 5) in response to local stimuli such as shade, nutrient availability, and moisture (see below); many organisms, and in particular the fungi and numerous bacteria, form spores. As any weightlifter knows, a plastic trait in humans is muscle mass.
Where flexibility is seasonally cyclic it is termed life cycle staging. Rock ptarmigan in the Canadian arctic camouflage themselves from predators by changing their plumage from white to dark to mottle in concert with the seasons. Where this form of plasticity differs from conventional polyphenism is in the further, reversible phenotypic variation that occurs within a male bird. When snow melts in the spring, females molt into their summer cryptic plumage, whereas males remain white for some time. As such, they are more conspicuous to females in competitive displays, evidently enhancing reproductive success.
The cost, however, is that they are also more conspicuous to predators. When females begin egg-laying, the males start to soil their plumage, and are maximally dirty when incubation begins (and their mates no longer fertilizable). Shortly thereafter the males molt to their cryptic summer plumage. This interpretation of events was developed from a careful, long-term study by Montgomerie et al. (2001) that systematically eliminated alternative hypotheses. In passing it is worth noting that here, and in related cases of seasonal coat coloration, one would expect strong selection for synchrony between organism and environment because mismatches (e.g., change in color of an animal to white in absence of snow) are striking and render the compromised individuals highly vulnerable. In those animals where cyclical polyphenism may be regulated by photoperiod, mismatches are a particular issue of increasing concern given prospective decreased snow duration as climates warm (Mills et al. 2013).
As suggested by its various manifestations summarized above and exemplified in Table 7.2, phenotypic plasticity offers the potential major advantage of dampening the effects of selection by uncoupling the phenotype from the genotype (Stearns 1982). In so doing it provides for adaptation to variable environments. While plasticity is generally viewed as expanding the capability of a given genotype, it also allows different genotypes to assume the same phenotype. At the population level, such plasticity may allow retention of genetic variation, analogous to dominance (Bradshaw 1965). Plasticity may also facilitate the origin of novel changes leading to speciation events and macroevolution (i.e., above the level of species, such as those affecting multiple families within a lineage; see examples in West-Eberhard 1989 and Pfennig et al. 2010).
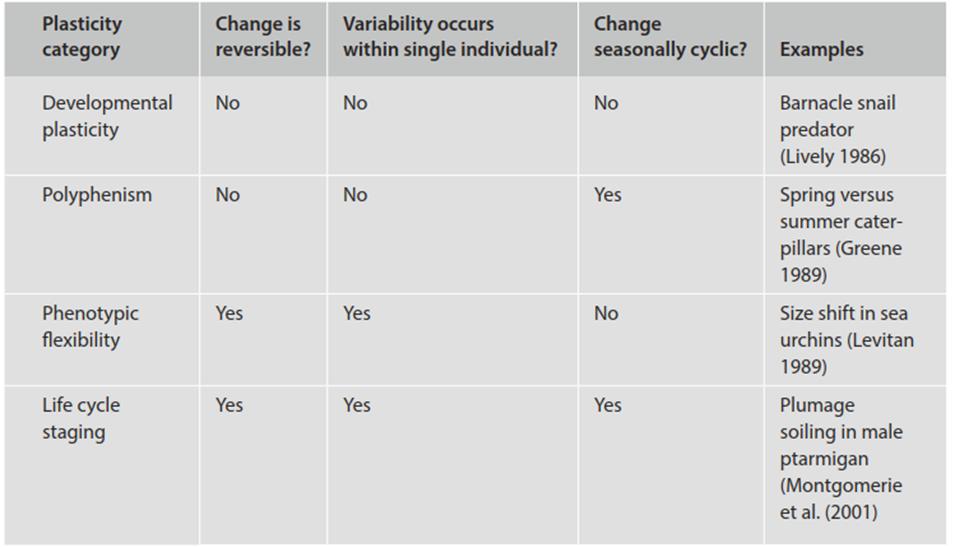
Table 7.2. Major forms of phenotypic plasticity (Piersma and Drent 2003, except where noted otherwise)
It is generally assumed intuitively that plasticity is adaptive but there have been relatively few direct tests (for one example, see Dudley and Schmitt 1996). There are costs and constraints to plasticity (see Table 7.3 and DeWitt et al. 1998; Givnish 2002; Valladares et al. 2007) and quantitative methods for assessing these in principle have been proposed (DeWitt et al. 1998). Plasticity cannot be invariably advantageous: No phenotype is favored in all environments and natural selection may favor fixed, plastic, or entirely novel phenotypes depending on the environmental context (Bradshaw 1965; Givnish 2002). The potential range in phenotypic variation is set genetically and is trait-specific (Bradshaw 1965). Hence, plasticity in some attributes is associated with and may enable stability in others.
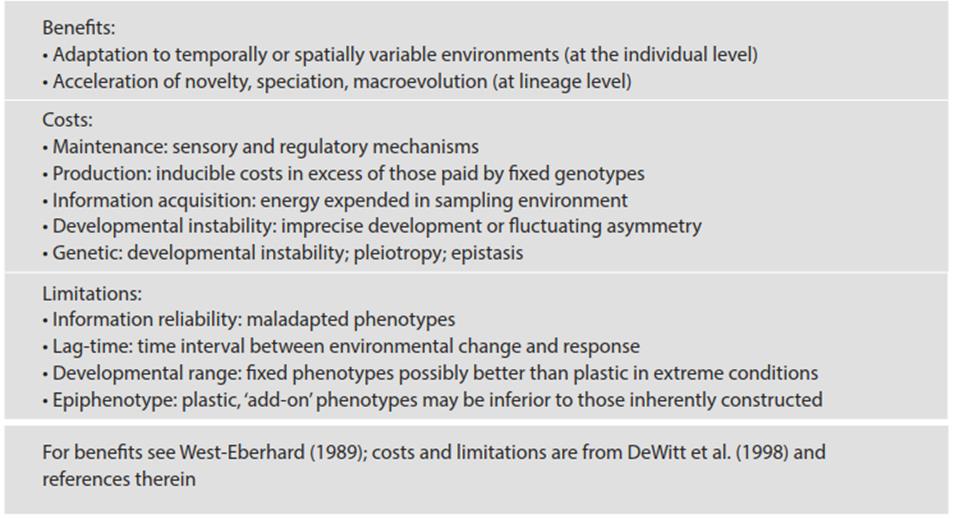
Table 7.3. Key potential benefits, costs, and limitations of phenotypic plasticity
Certain taxa, especially plants as already noted, and fungi (below), are more morphologically plastic than others, and modular organisms more so than unitary creatures. Within a taxon, seed size in plants and spore size in fungi are relatively fixed: A large as opposed to a small plant does not produce larger seeds, but rather more of them (for caveats see Thompson and Rabinowitz 1989). Within lineages the most common ultimate constraints on the extent of developmental polymorphisms probably are allometric (Chap. 4). For example, upward plasticity in birth weight cannot exceed a limit without increase in size of the adult; if adult size increases, an increase in birth weight will necessarily follow, whether it is adaptive or not (Stearns 1982).
Where selection is directional (i.e., favoring phenotypes at one end of the range), plasticity can extend the response of a population with limited genetic variability. However, it seems to be more important where selection is disruptive (i.e., favoring individuals at both extremes of a range). Whenever there is non cyclic, recurrent variation in time at intervals less than the generation time of the organism, adaptation can only be by plasticity (however, see later comments about mobile genetic elements; also comments later on environmental grain with respect to phenotypic or genotypic responses). Environmental conditions may change rapidly for desert plants exposed to sudden rainfalls or for microbes exposed to sporadic pulses of nutrients. Disruptive selection can also occur over short distances, as for the clonal plant whose stolons encounter varying degrees of competition, or the aquatic plant that roots in the littoral zone, grows up through the water column, and finally produces leaves and flowers above the surface (below).
Date added: 2025-06-15; views: 301;
